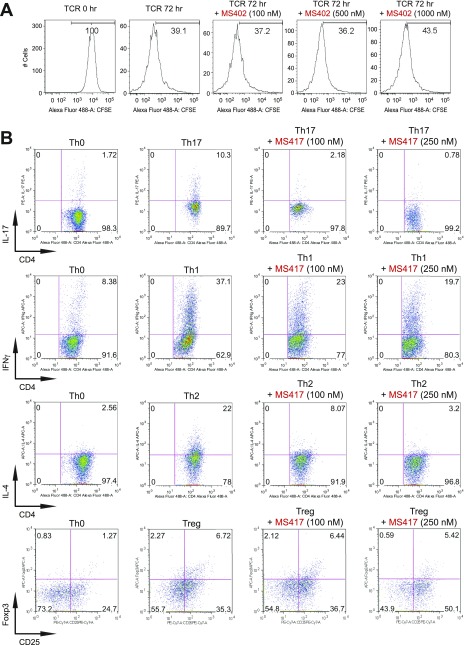
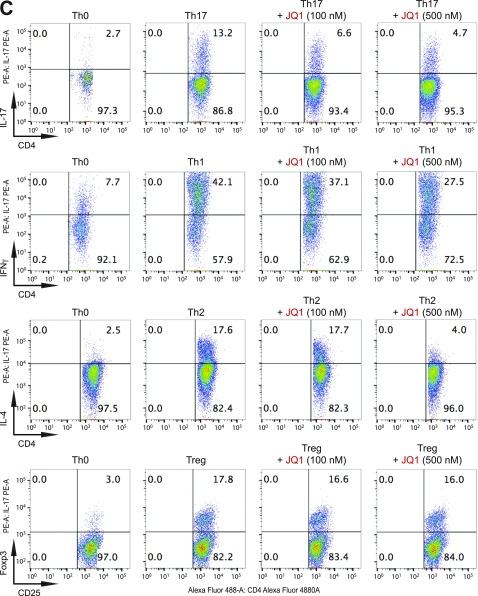
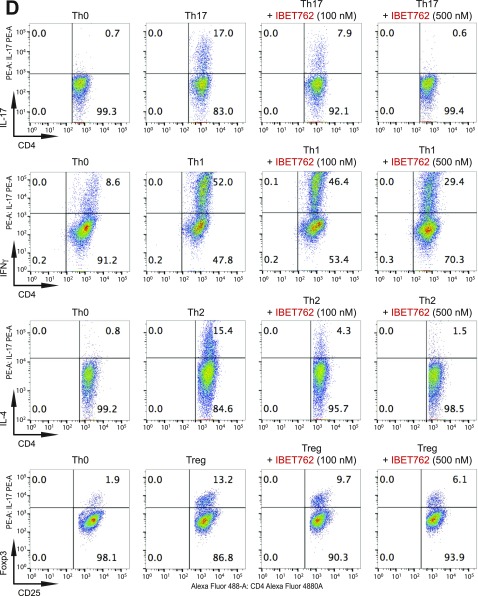
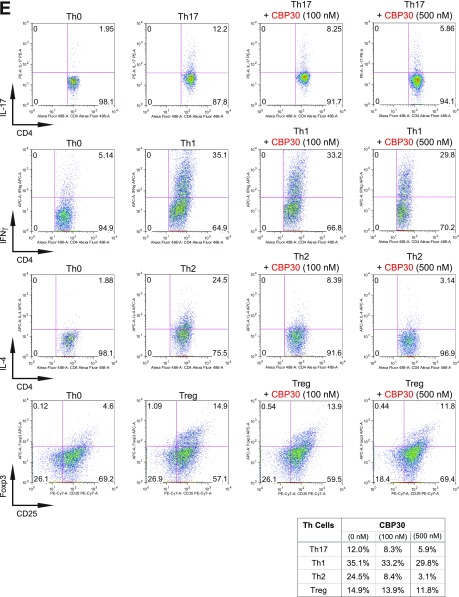
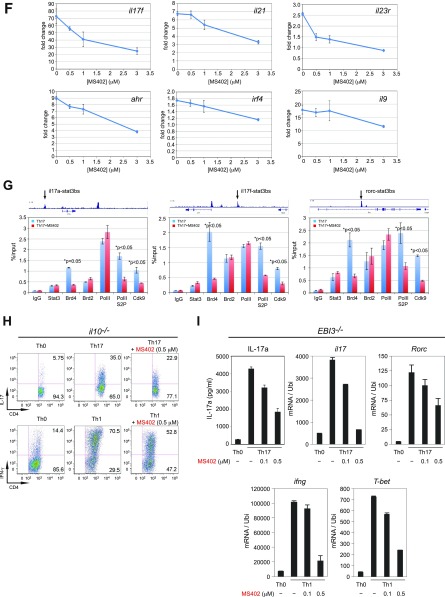
Fig. S2.
BET proteins control gene transcriptional programs in cell differentiation of T-helper cells. (A) Naive CD4+ T cells isolated from spleen and lymph nodes and stimulated to proliferate with plant-bound anti-CD3 plus anti-CD28 for 72 h under different concentrations of MS402. (B) Flow cytometry analysis of mouse primary naïve CD4+ T cells purified from spleens and lymph nodes of C57BL/6 mice and differentiated under Th0, Th1, and Th17 polarization conditions after 3 d with and without MS417 added daily at 100 nM or 250 nM. (C) Flow cytometry analysis of mouse primary naïve CD4+ T cells purified from spleens and lymph nodes of C57BL/6 mice and differentiated under Th0, Th1, and Th17 polarization conditions after 3 d with and without JQ1 added daily at 100 nM or 500 nM. (D) Flow cytometry analysis of mouse primary naïve CD4+ T cells purified from spleens and lymph nodes of C57BL/6 mice and differentiated under Th0, Th1, and Th17 polarization conditions after 3 d with and without IBET762 added daily at 100 nM or 500 nM. (E) Flow cytometry analysis of mouse primary naïve CD4+ T cells purified from spleens and lymph nodes of C57BL/6 mice and differentiated under Th0, Th1 and Th17 polarization conditions after 3 d with and without a selective CBP BrD inhibitor, SGC-CBP30 added daily at 100 nM or 500 nM. (F) Effects of MS402 treatment on mRNA expression levels of additional Th17-specifying transcription factors and cytokines after 3-d Th17-specific cell differentiation from mouse primary naïve CD4+ T cells in a dose-dependent manner. (G) ChIP-seq tracks of Stat3 on il17a-f and rorc gene loci in Th17 cells (Upper) and ChIP-qPCR analysis of Stat3, Brd4, Brd2, PolII, PolIIS2P, and Cdk9 in Th17 cells treated with and without MS402 (3 μM) (Lower). Statistically significant (*P < 0.05) results are annotated. All results are representative of more than two independent experiments. (H) (Upper) Naïve CD4+ T cells of il10−/− mice were differentiated under Th0 and Th17 polarizing conditions. Th17 cells were treated with or without MS402 (500 nM). After in vitro culturing for 3 d the cells were restimulated with PMA/ionomycine for 6 h, stained for intracellular IL-17 for flow cytometry analysis. (Lower) A similar experiment was performed under Th1 polarizing conditions to assess MS402’s effect on IFN-γ–producing CD4+ cells. (I) Naive CD4+ T cells isolated from EBI3−/− mice were differentiated under Th1 or Th17 polarizing conditions. Th17 cells were treated with or without MS402 added daily at concentration of 100 or 500 nM. After in vitro culturing for 3 d the cells were restimulated with PMA/ionomycine for 6 h, and real-time PCR was performed for detection of mRNA expression of Th17 and Th1 cytokines and transcription factors. In addition, supernatants were harvested and IL-17 production was analyzed by ELISA.