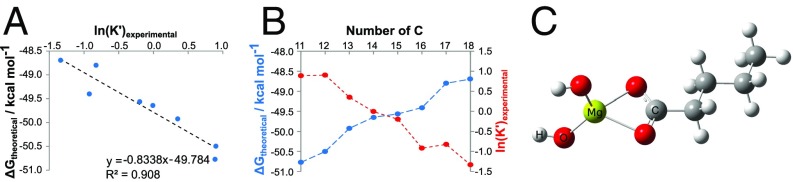
Fig. 3.
Characterization of dihydroxymagnesium carboxylates. (A) The negative correlation between the experimental equilibrium constant, expressed as ln(K′), and the computed Gibbs free energy ΔG of the [(OH)2MgO2CCnH2n+1]− complex formation with n ∈ ℕ for different linear alkyl chain lengths between C11 and C18, as computed with density functional theory (DFT). (B) The dependency of ln(K′) (experimentally via MS) and ΔG (theoretically via DFT) on different alkyl chain lengths is displayed to illustrate the reactivity of dihydroxymagnesium carboxylates, whereas the optimized computed geometry for the representative ion dihydroxymagnesium-n-pentanoate [(OH)2MgO2CC4H9]− is depicted in C (see Table S2 for the computed coordinates of relaxed geometry of C5-dihydroxymagnesium carboxylate complex anion).