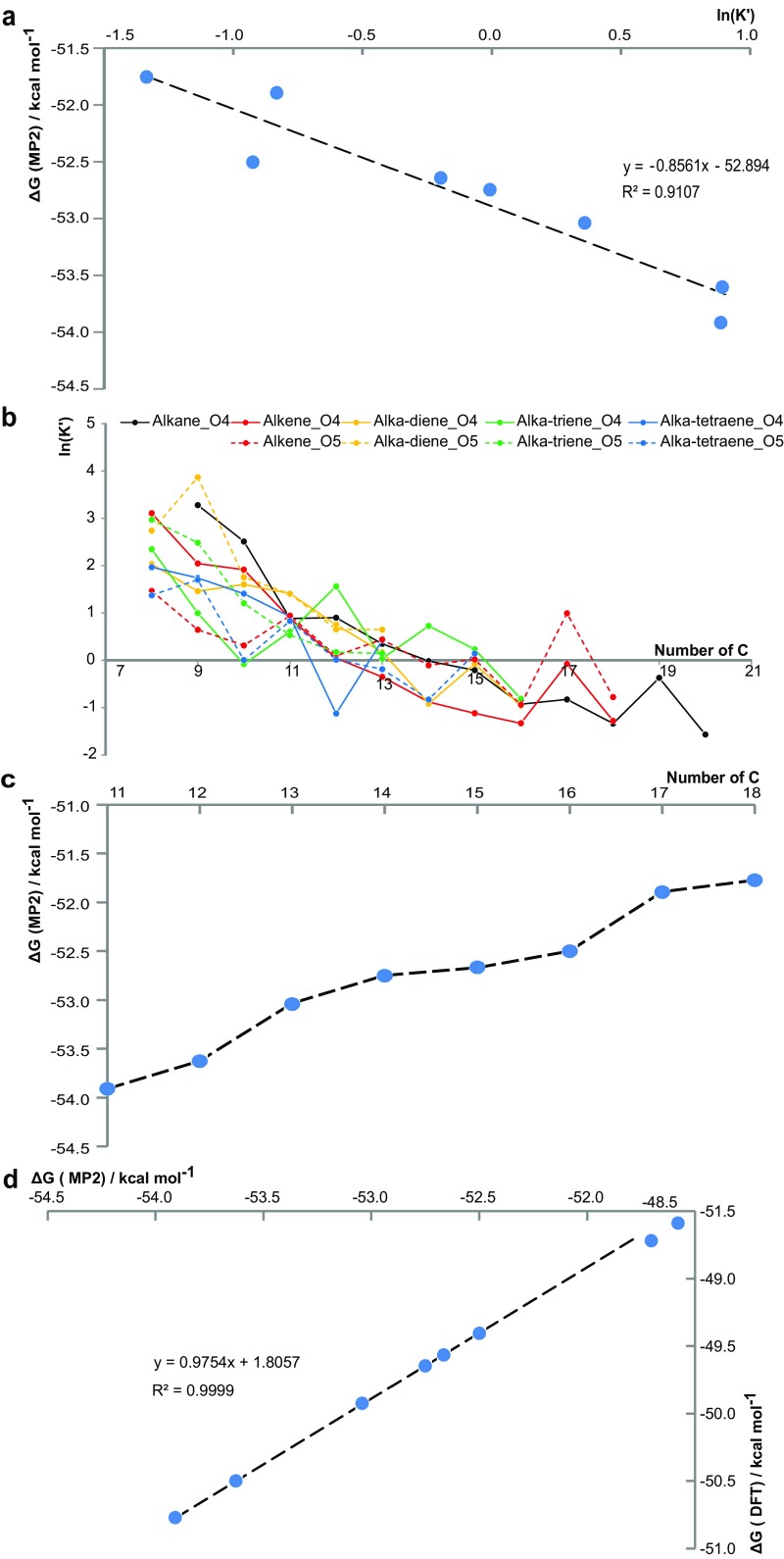
Fig. S5.
General CHOMg reactivity and verification of DFT simulations with MP2 level of theory. (A) Negative correlation of the Gibbs free energy ΔG with ln(K′), following Eq. 2 for different linear alkyl chain lengths between C11 and C18, computed with MP2 extracts (discussed in the main text). (B) ln(K′) is plotted vs. the number of carbon atoms for several homologous series, varying by alkyl saturation and number of oxygen atoms in the aliphatic chain. A general decreasing trend with increasing numbers of C atoms is observed, indicating that smaller alkyl chain CHO molecules are more reactive to form CHOMg compounds, relatively longer aliphatic chain molecules. Additionally, local reactivity anomalies are highlighted by functional fluctuations. (C) ΔG is plotted vs. the number of carbon atoms in linear alkyl chain lengths of the [(OH)2MgO2CCn]− complex formation with n ∈ , as computed on MP2-level of theory. (D) Correlation between the DFT-B3LYP and MP2 methods, which illustrates the accuracy of DFT, describing this complex formation reaction properly.