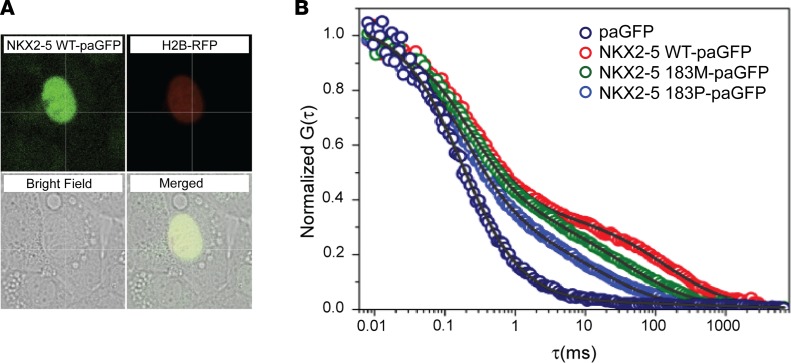
Figure 1. NKX2-5 mutant protein displays decreased affinity for its endogenous DNA binding sites.
In situ analysis of DNA binding in transiently transfected HL-1 cardiomyocytes confirms decreased affinity of Nkx2-5 I184M and P for target sites. (A) Example of nucleus analyzed by photoactivation; H2B-RFP fluorescence (red) colocalizes with Nk2-5-paGFP–transcfected (green) cells, while GFP can only be visualized after photoactivation. (B) Typical autocorrelation curves for paGFP and NKX2-5 proteins (WT, 183M, and 183P), showing fast and slow diffusion components for each protein. Note anomalous diffusion for NKX2-5 mutant proteins. pa, photoactivatable; RFP, red fluorescent protein; G, fluctuations in fluorescence; τ, time in milliseconds.