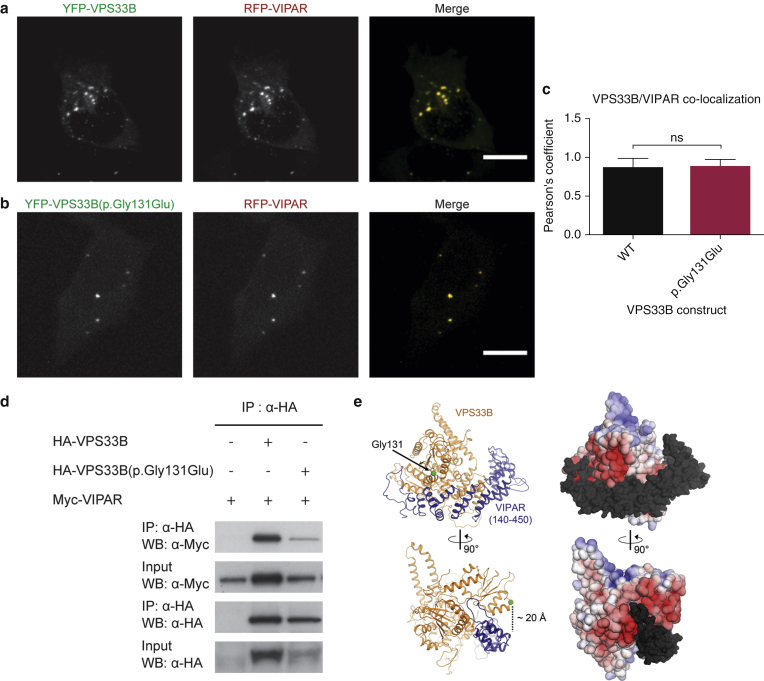
Figure 2.
VPS33B(p.Gly131Glu) does not affect the VPS33B-VIPAR interaction. (a, b) Representative mIMCD3 cells coexpressing RFP-VIPAR and (a) wt YFP-VPS33B or (b) YFP-VPS33B(p.Gly131Glu). Scale bars = 10 μm. (c) Quantification of colocalization between VPS33B constructs and VIPAR. Data are mean ± standard deviation, unpaired t-test, ns = nonsignificant. (d) Anti-HA co-IP on HEK-293 cell lysates coexpressing HA-VPS33B or HA-VPS33B(p.Gly131Glu) with Myc-VIPAR. (e) Mapping of p.Gly131Glu mutation on structural models of the VPS33B-VIPAR complex. Left: VPS33B in orange and alpha solenoid region of VIPAR (residues 140–450) in blue; the disordered N-terminus of VIPAR is omitted. VPS33B residue Gly131 is a green sphere. Right: surface potential of VPS33B, from −5 kbTec−1 (red) to +5 kbTec−1 (blue), VIPAR is dark gray. co-IP, co-immunoprecipitation; HA, hemagglutinin; HEK, human embryonic kidney; IP, immunoprecipitation; mIMCD3, murine inner medullary collecting duct 3; RFP, red fluorescent protein; WB, western blot; wt, wild type; YFP, yellow fluorescent protein.