Figure 2. Evaluation of 5E5 CAR T Cells Reactivity to a Panel of Human Primary Cells.
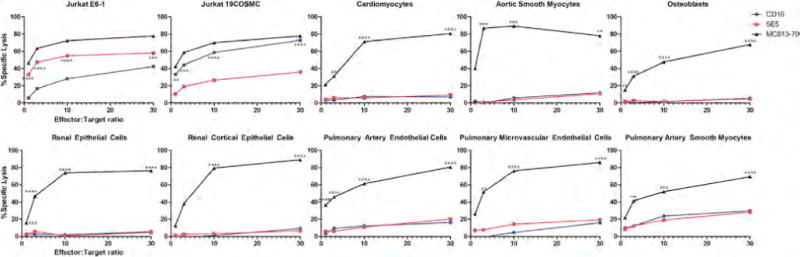
5E5 and CD19 CAR T cells were tested in a chromium release lysis assays at effector:target ratios of 1:1 to 30:1. MC813-70 CAR T cells known to exhibit normal tissue toxicity were used as a positive control. In addition the various CAR T cells were tested on wild-type Jurkat and Jurkat with reconstituted COSMC. Statistical comparisons are between 5E5 CAR and CD19 CAR in the positive controls Jurkat E6-1 and Jurkat 19COSMC. All other comparisons are made between MC813-70 and 5E5. ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001.
