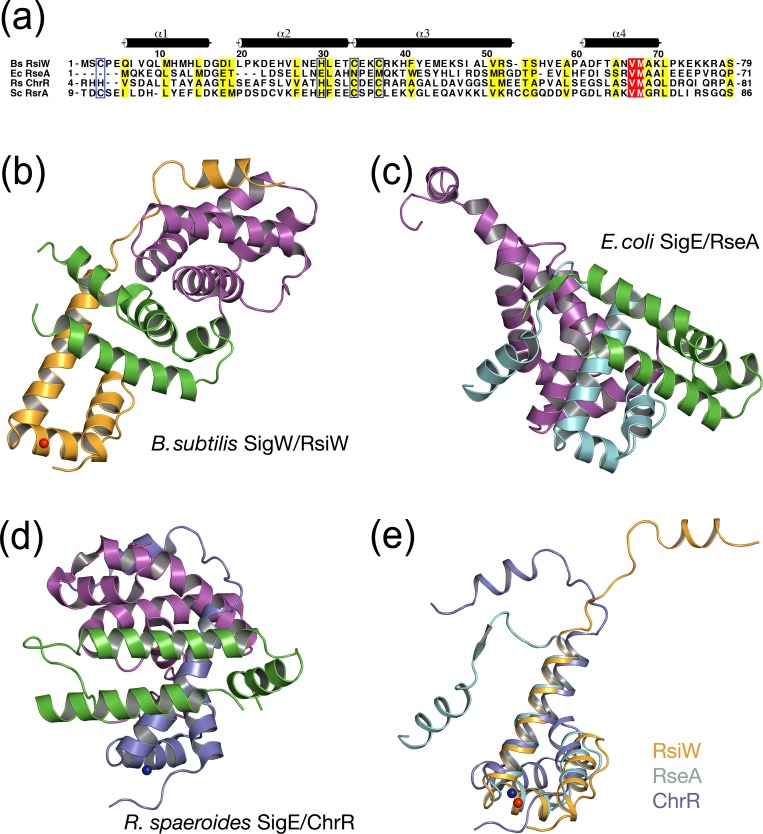
Fig 6. Structural comparison of group IV sigma/anti-sigma factors.
(a) Sequence alignment of anti-sigma factors. Species abbreviations are as follows: Bs, B. subtilis; Ec, E.coli; Rs, R. sphaeroides; Sc, S. coelicolor. The residues aligned with a CHCC in Bs RsiW are marked with blue boxes. Coordinates of Ec RseA and Rs ChrR used in the structure alignment were obtained from Protein Data Bank (PDB IDs: 1OR7 and 2Z2S). (b-d) Ribbon models of the complexes. σ2 and σ4 in sigma factors are colored magenta and green, respectively. Anti-sigma domains of RsiW (b), RseA (c), and ChrR (d) are colored orange, teal, and purple, respectively. The models are drawn in the same orientation after N-terminal helical bundles (α1’-α3’) of anti-sigma are superimposed. (e) The superimposition of anti-sigma domains. The positions of the C-terminal motifs are variable, depending on the binding of the cognate sigma factor.