Figure 4.
Structural Determinants of Flexibility within the N-terminal Hairpin Module.
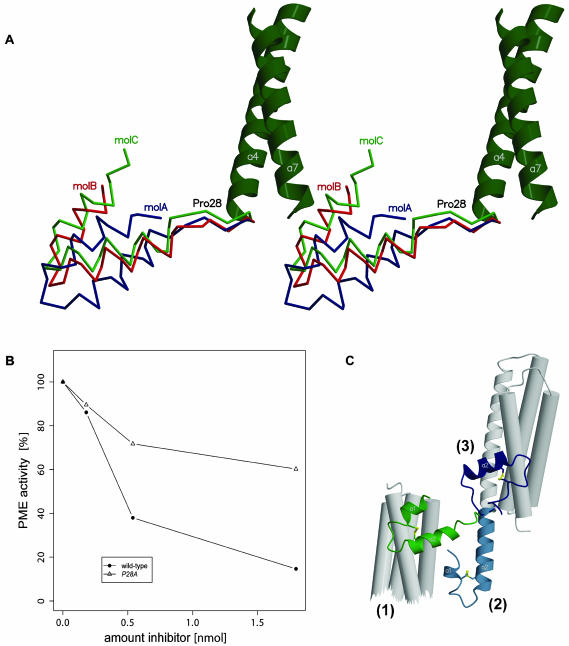
(A) Stereo view of the three PMEI molecules in the asymmetric unit, superimposed with respect to the four-helix bundle. The relative displacement indicates conformational variability. Note that the difference in orientation between molA and molC is almost 90°.
(B) Decreased inhibitory power of the PMEI P28A mutant in comparison with the wild-type inhibitor (solid circle) in plant PME inhibition assays.
(C) Structural superposition of the wild-type PMEI dimer (green) and two P28A mutant structures shown in dark (form A) and light blue (form B) highlight conformational flexibility of the PMEI hairpin. The flipped-out state (2) might resemble an intermediate in transition between dimer (1) and monomer (3).