Abstract
Background
Accumulating data point to intermediate-conductance calcium-activated potassium channel (IKCa1) as a key player in controlling cell cycle progression and proliferation of human cancer cells. However, the role that IKCa1 plays in the growth of human cervical cancer cells is largely unexplored.
Material/Methods
In this study, Western blot analysis, immunohistochemical staining, and RT-PCR were first used for IKCa1protein and gene expression assays in cervical cancer tissues and HeLa cells. Then, IKCa1 channel blocker and siRNA were employed to inhibit the functionality of IKCa1 and downregulate gene expression in HeLa cells, respectively. After these treatments, we examined the level of cell proliferation by MTT method and measured IKCa1 currents by conventional whole-cell patch clamp technique. Cell apoptosis was assessed using the Annexin V-FITC/Propidium Iodide (PI) double-staining apoptosis detection kit.
Results
We demonstrated that IKCa1 mRNA and protein are preferentially expressed in cervical cancer tissues and HeLa cells. We also showed that the IKCa1 channel blocker, clotrimazole, and IKCa1 channel siRNA can be used to suppress cervical cancer cell proliferation and decrease IKCa1 channel current. IKCa1 downregulation by specific siRNAs induced a significant increase in the proportion of apoptotic cells in HeLa cells.
Conclusions
IKCa1 is overexpressed in cervical cancer tissues, and IKCa1 upregulation in cervical cancer cell linea enhances cell proliferation, partly by reducing the proportion of apoptotic cells.
MeSH Keywords: Cell Proliferation, Clotrimazole, Uterine Cervical Neoplasms
Background
Worldwide, cervical cancer is the third leading cause of cancer-related mortality among women [1]. A major risk for the development of cervical cancer is a persistent infection with human papilloma virus (HPV), which can lead to squamous cell carcinoma (SCC) and cervical adenocarcinoma (AD) [2]. However, HPV alone is not sufficient to immortalize and transform the epithelial host cells [3]. Other molecular factors that regulate the growth of the cells and contribute to cervical carcinogenesis pathways are essential. Previous research found that potassium channels play a role in the regulation of cell proliferation and differentiation [4,5]. Unregulated cell proliferation and uncontrolled differentiation are strongly associated with tumorigenesis.
The IKCa1channel, also known as KCa3.1 or SK4, is a product of the KCNN4 gene. The IKCa1channel-forming subunit is constitutively associated with calmodulin, thus making these channels strictly Ca2+-dependent [6]. IKCa1 channels are expressed in a number of tissues and play diverse physiological roles, including the regulation of intracellular calcium homeostasis [7–9]. In recent years, IKCa1 channels have been implicated in carcinogenesis after studies demonstrated this channel to be upregulated in several solid cancers, including prostate cancer, hepatocellular carcinoma, endometrial, mammary carcinoma, and glioblastoma [10–15], and in several cancer cell lines [16,17]. Further studies found that the mechanism underlying the upregulation of IKCa1-mRNA in cancer cells was due to the activation of mitogen-activated protein (MAP) kinase signaling and the resultant AP-1-mediated mRNA transcription [18–20]. Physiologically, IKCa1 channels are used to modulate K+ efflux and hyperpolarization following channel activation by the release of Ca2+ from intracellular stores. Through a positive feedback mechanism, IKCa1 channels act as a cell-membrane hyperpolarizing and countercurrent-producing channel [24,25]. These channels regulate anion and water secretion in the gut [21], endothelium-derived hyperpolarization-mediated vasodilation [22], cell volume [23], and Ca2+ dynamics. IKCa1 participates in cell proliferation, as demonstrated by several studies that have shown IKCa1-function to be required for Ca2+-sensitive steps of cell cycle progression. Cells in hyperpolarized state due to K+ channel activation exhibit enhanced calcium entry and calcium homeostasis, which is critical in controlling the passage of cells through G0/G1 or the G1/S phase transition [26,27]. Pharmacological blockade or genetic reduction of IKCa1 in vitro increases p21Waf1/Cip1 expression and decreases the expression of cyclin E, which suppresses proliferation of pancreatic cancer and hepatocellular carcinoma cells [12,17]. TRAM-34, a specific IKCa1 blocker, can suppress cellular growth [10]. Together, these studies support that IKCa1 could be potential molecular marker for tumor growth and tumor progression, as well as a potential treatment target [14,28,29]. However, the impact of IKCa1 on the growth of human cervical cancer cells is unknown.
In this study, we determined the expression level of IKCa1 in cervical cancer tissues and investigated its role in cell proliferation and apoptosis. We found that IKCa1 is highly expressed in cervical cancer tissue and that the IKCa1 channel blocker, clotrimazole, and IKCa1 channel siRNA inhibit the growth of cervical cancer HeLa cells. This was associated with a decrease of IKCa1 mRNA expression and IKCa1 channel current, as well as the increase in the proportion of apoptotic cells. These findings provide support for targeting IKCa1 channels in a therapeutic strategy for treatment of cervical cancer.
Material and Methods
Cervical cancer samples
We collected 30 cervical cancer tissues (CC) from patients in the Affiliated Hospital of Southwest Medical University during the years 2013 and 2014. Tissues originated from patients ages 30 to 51 years old, with a median age of 41. As controls, we used 18 normal cervical tissues (NC) obtained from patients ages 42 to 60 years old, with a median of 51, during surgery for benign disease (uterine fibroids or uterine adenoma). No patient received radiotherapy or chemotherapy before the operation. Cervical cancers were staged in 9 patients as stage I, in 11 as stage II, in 6 as stage III, and in 4 as stage IV. Pathological examination of 30 cervical cancer cases were classified into 5 cases of G1, 20 cases of G2, and 5 cases of G3.
Ethics statement
Human tissue collection was performed by the Affiliated Hospital of Southwest Medical University. All patients gave informed written consent and the study was approved by the local government.
Cell culture
Human cervical cancer cell line HeLa and cervical epithelial cell line H8 were bought from the Department of Pathophysiology of Chongqing Medical University, and maintained as subconfluent monolayers in RPMI-1640 supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Cells were cultured in an incubator at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The culture medium was changed every 2 days.
RNA extraction, reverse transcription (RT), and PCR
Total RNA was extracted from tissues and cells using TRIzol™ reagent (Invitrogen) following the manufacturer’s protocol. First-strand cDNA was synthesized using the Revert AidTM First-Strand cDNA Synthesis Kit. For semi-quantitative RT-PCR, GAPDH and β-actin were used as the internal reference and were co-amplified with the target gene in every PCR reaction. Primers for RT-PCR analysis were designed as follows: GAPDH (forward, 5′-ATGCTGGCGCTGAGTACGTC-3′, reverse, 5′-GGTCATGAGTCCTTCCACGATA-3′); β-actin (forward, 5′-CTCC ATCCTGGCCTCGCTGT-3′, reverse, 5′-GCTGTCACCTTCACCGTTCC-3′); IKCa1 (forward, 5′-GTGCGTGCAGGATTTAGGG-3′, reverse, 5′-TGCTAAGCAGCTCAGTCAGGG-3′). Amplification was conducted in the following conditions: 30 cycles of 4 min initial denaturation at 94°C, then 94°C for 30 s, 57°C for 45 s, and 72°C for 1 min, and a final 10-min extension termination at 72°C. The PCR products were made on 2% (w/v) agarose gel containing ethidium bromide and then visualized under ultraviolet trans-illumination. Then, the optical density value of electrophoresis strips was calculated. The ratio of IKCal to GAPDH represents the relative IKCa1 mRNA expression level.
Immunohistochemical analysis
Cervical cancer specimens and cervical tissues from the surgical patients with benign disease were fixed in 10% neutral-buffered formalin, embedded in paraffin, and thin-sectioned at 2 μm [30]. Immunohistochemical staining of sections was done using streptomycin avidin–peroxide (SP) enzyme. A rabbit polyclonal antibody for IKCa1(ADL Company) was used as the primary antibody. Goat-anti-rabbit polyclonal antibody labeled with horseradish peroxidase (Beijing Zhongshan Golden Bridge Biotechnology Co., LTD) was used as the secondary antibody. To assess histological differences between cancer tissues and normal tissues, 1000 cells were selected at random and examined by light microscopy for each section. A positive result was when the cells cytoplasm or nucleus stained brown and appeared as granules. Staining intensity and the percentage of positive cells/total cells were semi-quantitatively calculated [31].
Medicine intervention on HeLa cells assay
An IKCa1 channel blocker, clotrimazole (Sigma) was used to test the effect on HeLa cell proliferation. HeLa cells were divided into an experimental group and a control group. HeLa cells were placed in 24-well cell culture plates (10 000 cells per well) and were grown overnight. The following day, the serum from the cells in the experimental group was removed and replaced with serum-containing media including 5, 10, 20, and 40 μM of the inhibitor clotrimazole. Anhydrous ethanol instead of inhibitor-containing serum was added to the wells that contained cells of the control group. To prevent degradation of the inhibitor, inhibitor-containing media was freshly prepared and replaced the old serum every 24, 48, and 72 h after first application of inhibitor. Cell numbers were quantitatively assessed using a Coulter Counter. For both groups, a cell growth curve was recorded. Cell proliferation was determined by MTT method. We used RT-PCR to determine mRNA levels of IKCa1 and used patch clamp recordings to measure IKCal channel current.
Cell proliferation assay
Cell proliferation rates were quantified using colorimetric MTT proliferation assay. HeLa cells were placed in 96-well cell culture plates at the density of 8000 cells per well and were grown overnight. Cells were exposed to different concentrations clotrimazole or ethanol and grown for 24, 48, and 72 h. Cells were washed with basal solution containing: 135 mM NaCl, 3.8 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, 1.2 mM KH2PO4, 10 mM HEPES, and 10 mM glucose (pH=7.4, adjusted with NaOH). Aliquots of basal solution containing 0.5 mg/ml thiazolyl blue tetrazolium bromide (MTT, Sigma-Aldrich) were added to each well and incubated at 37°C MTT for 4 h. Then, the solution was removed, cells were solubilized in 150 μl dimethyl sulfoxide to dissolve newly formed MTT-formazan particles, and incubated at 37°C for 10 min. Next, culture plates were gently agitated and cell proliferation was measured by examining absorbance at 490 nm using an ELx800 Absorbance microplate reader (BioTek Instruments, Winooski, VT, USA). The rate of cell proliferation inhibition (“R”) was calculated using the equation “R=[(A0−At)/A0]×100%”. “A0” is the average absorbance value of the control group. “At” is the average absorbance value of the experimental group.
Patch clamp electrophysiology
Conventional whole-cell patch clamp technique was used to measure and record IKCa1 currents [32]. HeLa cells were planted in 25cm2 cell culture flasks at the density of 1×106 cells per flask and were grown in RPMI1640 complete culture solution for 24 hours. HeLa cells were harvested with 1% trypsin/EDTA then divided into a control group whose cells grown incomplete culture solution without clotrimazole and experimental group treated with 20μM clotrimazole. Cells were then grown for another 48 hours. Cells were briefly washed with PBS and digested with 1% trypsin/EDTA solution to produce single-cell suspension. The single-cell suspension was planted on a Polylysine glass slide and was incubated at 37°C in RsBiotech for 1 hour. Cells used for the patch clamp experiment were selected based upon normal cellular morphology in the center of the slide. Whole-cell currents were recorded using an EPC-29-USB amplifier (HEKA, Lambrecht-Pfalz, Germany). Readings were possible due to the negative pressure suction exerted by the electrode of the patch clamp that was pressed to the cell surface and produces a resistance of 1−4GΩ, and with a compensating series resistance that offsets the capacitive current after the membrane rupture. Data were analyzed with Axon “pCLAMP” software.
IKCa1 siRNA transfection
Kca3.1 siRNA transfection was carried out using Lipofectamine 2000 reagent (Invitrogen, CA) according to the manufacturer’s instructions. siRNA oligonucleotide was synthesized by Wuhan Cell Marker Biotechnology Co., Ltd. siRNA sequences are as follows, IKCal1 (Sense strand, 5′-CACCGCCTGGATGTTCT ACAAACATTTCAAGACGATGT TTGTAGAACATCCAGGCTTTTTTG-3′, antisense strand, 5′-AGCTCAAAAAAGCCTGGATGTTCT ACAAAC ATCGTCTTGAAATGTTTGTAGAACATCCAGGC-3′); negative control (Sense strand, 5′-CACCGCCTGATGACATTGACTTACATTCA AGACGTGTAAGTCAATGTCATCAGGCTTTTTTG-3′, antisense strand, 5′-AGCTCAAAAAAGCCTGATGACATTGACTTACACG TCTTGAATGTA AGTCAATGTCATCAGGC-3′). Three different conditions were used for the siRNA experiment. IKCal siRNA group transfected with pGenesil-IKCa1 siRNA+Lipo, negative control group with pGensil-HK+Lipo, and blank with RPMI-1640 + Lipo. pGensil-HK is a negative control plasmid and Lipo stands for Lipofectamine 2000 reagent. Cells were grown for an additional 48 hours following transfection then protein levels, mRNA levels, cell proliferation, cell apoptosis and channel current of IKCal were assessed.
Apoptosis analysis
Cell apoptosis was assessed using the Annexin V-FITC/Propidium Iodide (PI) double-staining apoptosis detection kit (KeyGen, China). The cells were collected and stained according to the manufacturer’s instructions. The apoptosis data acquisition and analysis was performed by a FACS Calibur flow cytometer. Basal apoptosis was identically determined on control cells.
Western blot
The HeLa cells were homogenized on ice and the cell lysates were prepared through centrifugation at 14000×g for 10 min at 4°C. Total protein concentration was quantified using the bicinchoninic acid assay (BCA, Pierce). Equal amounts of protein were loaded and separated using SDS-PAGE and then transferred onto a nitrocellulose membrane. The blots were blocked with 10% non-fat milk and incubated with the rabbit-anti-human polyclonal IKCa1 antibody (1: 500) overnight at 4°C. The next day, the membrane was washed with PBS 3 times and incubated with horseradish peroxidase-conjugated secondary antibody (1: 2000) at 37°C for 1 h. The Odyssey infrared fluorescent scanning system (LICOR) and Odyssey V1.2 software were used to quantify the expression of KCa3.1. The ratio of IKCa1 and β-actin (loading control) optical density were used to measure the relative expression of IKCa1 protein.
Statistical analysis
All statistical analysis was done using SPSS 13.0 software. For comparison of 2 data sets, we used the t test and multiple comparisons were analyzed using a Kruskal-Wallis H test. The Spearman rank correlation test was used for correlation analysis. Data used for statistical analysis are expressed as the mean ±SE. The differences among groups were determined using ANOVA. P<0.05 was considered statistical significance.
Results
Given their demonstrated importance in cell proliferation, we hypothesized that the intermediate-conductance-Ca2+-activated K+ channel may participate in the regulation of cervical cancer proliferation. To test the involvement of IKCa1 channels in cell proliferation, we performed experiments to determine whether there were changes in mRNA and protein in patients with cervical cancer. We used downregulated IKCa1 gene expression in HeLa cells by an IKCa1 siRNA transfection or by an IKCa1 blocker and measured the effect on mRNA expression, protein levels, cellular proliferation, and apoptosis.
IKCa1 expression is increased in cervical cancer tissues
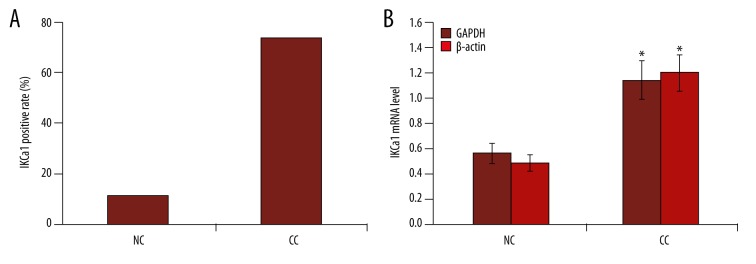
IKCa1 expression was examined in 30 cervical cancer tissues and 18 normal cervical tissues. As shown in Figure 1, 22/30 cervical cancer tissue cases were positive for IKCa1 mRNA (73.33% positive rate). Real-time PCR analysis showed that using GAPDH as internal reference, the average expression of IKCa1 transcripts in cervical cancer was 1.14±0.45. Only 2/18 normal cervical tissues displayed altered IKCa1 mRNA expression (11.11% positive rate). The average expression levels of IKCa1 mRNA were 0.56±0.23. We observed a statistically significant 2-fold higher mRNA expression in cervical cancer tissue compared to the normal cervical tissues. We got a similar result with β-actin as an internal reference (Figure 1). Statistical analysis also indicated a significant difference between the positive rates of cervical cancer and the normal cervical tissues (p<0.05).
Figure 1.
Semi-quantitative RT-PCR analysis of IKCa1 mRNA in cervical cancer tissues and normal cervical tissues. Mean ±SE is shown. IKCa1 gene expression was calculated relative to the reference gene GAPDH and β-actin. (A) Comparison of positive rate of KCa3.1 gene expression in tumor tissues from cervical cancer and normal cervical tissues. (B) Comparison of IKCa1 gene expression between tumor tissues from cervical cancer and normal cervical tissues. NC, normal cervical tissues. CC, cervical cancer tissues. * p<0.05.
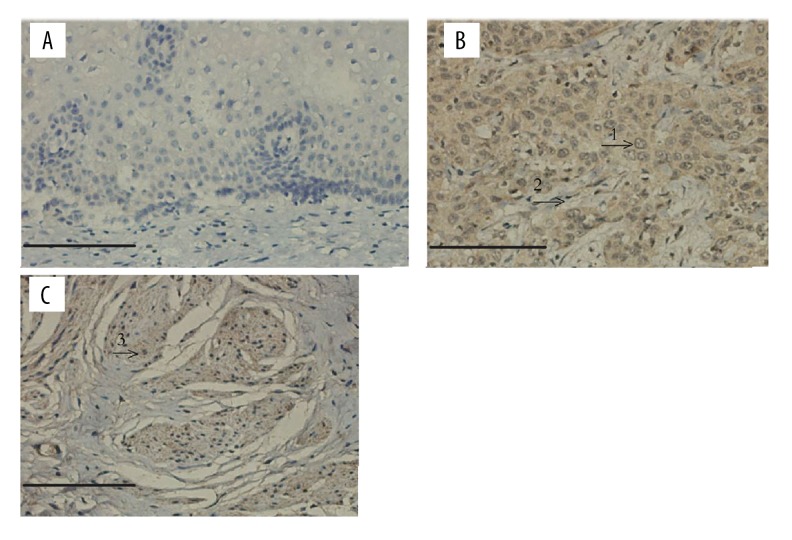
Immunohistochemistry analysis revealed that the expression of IKCa1 proteins was rarely detected in normal cervical tissues (only a few brown particles in 1 tissue sample; Table 1). In normal cervical tissues, IKCa1protein localized predominantly to the cell membrane and not to the cell nucleus. In contrast, we observed different amounts of brown particles with varying intensity of staining in all 30 of cervical cancer tissues (100% for positive rate). In 3/5 G1 cervical cancer tissue samples, we observed low expression of IKCa1 protein, and 1/5 appeared to express a moderate amount of IKCa1 protein. There was moderate expression in 18 out of 20 samples of G2 cervical cancer tissues and only 2 had low expression. As histological grade increased, the expression of IKCa1protein also increased. We observed strong expression of IKCa1 protein in 4/5 cases of G3 cervical cancer tissues and we observed moderate expression in 1/5 tissue samples (Table 1). Stained particles in the cervical cancer cells mainly localized in the cell nucleus and on the cell membrane (Figure 2). There was no correlation between IKCa1 expression in cervical cancer tissues with different clinical features of disease (r=0.176, P=0.352), but there was a significant correlation between IKCa1 expression and histological grade (r=0.736, P=0.000) (Table 1).
Table 1.
immunohistochemical analysis of IKCa1protein in cervical cancerand normal cervical tissues.
| Clinical features of disease | IKCa1 | Total | Positive rate (%) | P Value | |||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ||||
| NC | 17 | 1 | 0 | 0 | 18 | 5.56 | P=0.000 |
| CC | 0 | 5 | 21 | 4 | 30 | 100.00* | z=−6.109 |
| Histological grade | |||||||
| G1 | 0 | 3 | 2 | 0 | 5 | 100.00* | P=0.000 |
| G2 | 0 | 2 | 18 | 0 | 20 | 100.00* | χ2=16.468 |
| G3 | 0 | 0 | 1 | 4 | 5 | 100.00* | |
| Surgical disease stage | |||||||
| I–II | 0 | 9 | 7 | 4 | 20 | 100.00* | P=0.104 |
| III–IV | 0 | 2 | 6 | 2 | 10 | 100.00* | z=−1.624 |
“−” – no IKCa1protein; “+” – low expression of IKCa1protein; “++” – moderate IKCa1 expression; “+++” – strong IKCa1 expression; NC – normal cervical tissues; CC – cervical cancer tissues. The quantity of the Z value represents the distance between the raw score and the average.
p<0.05.
Figure 2.
Immunohistochemical staining for IKCa1 in tissues. (A) Immunohistochemical staining for IKCa1 in normal cervical tissues. (B, C), immunohistochemical staining for IKCa1 in cervical cancer tissues revealed a strong staining in cells that were primarily distributed in the cell nucleus and on cell membranes (arrows). “1”: IKCa1-positive expression in epithelial cells; “2”: IKCa1-positive expression in mesenchymal cells; “3”: IKCa1-positive expression in muscle cells. Original magnification, ×200. (Scale bars, 20 μm.)
IKCa1 is expressed in HeLa cells
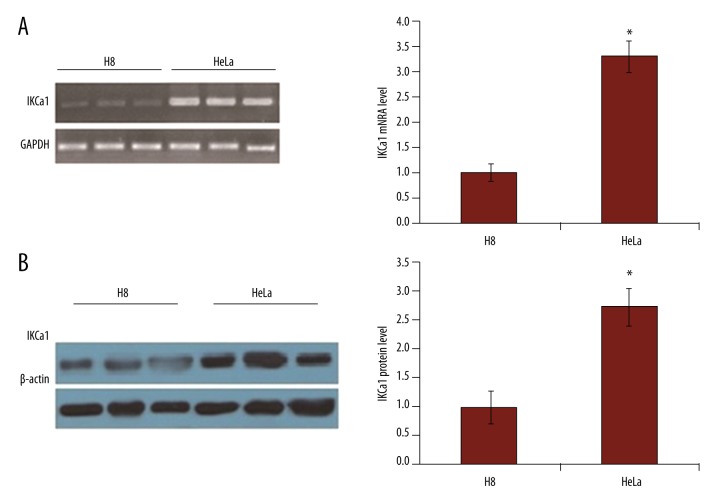
We used RT-PCR and Western blot analysis to determine if the IKCa1channel mRNA and protein were expressed in HeLa cells. As shown in Figure 3A and 3B, IKCa1 mRNA and protein levels were significantly elevated in HeLa cells compared to immortalized human cervical epithelial cells H8.
Figure 3.
The expression of IKCa1 mRNA and protein are elevated in HeLa cells. (A) RT-PCR was used to detect IKCa1 mRNA expression with GAPDH as a loading control. (B) IKCa1 protein expression was detected using Western blot in HeLa cells with β-actin used as a loading control. In both A and B (right), mRNA or protein levels were quantified by measurement of the optical density of the samples compared to the optical density of the loading controls. Mean values ±SE of gene or protein expression from 3 independent cell preparations for HeLa and H8. * P<0.05.
Clotrimazole inhibits the growth of HeLa cells
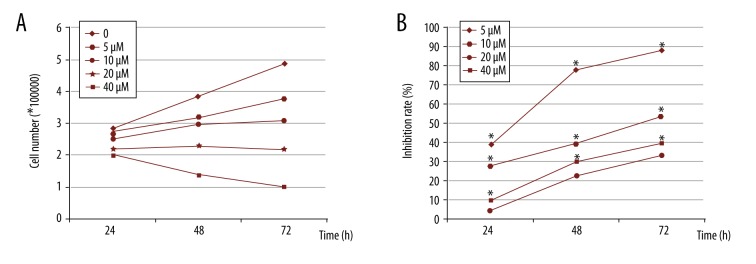
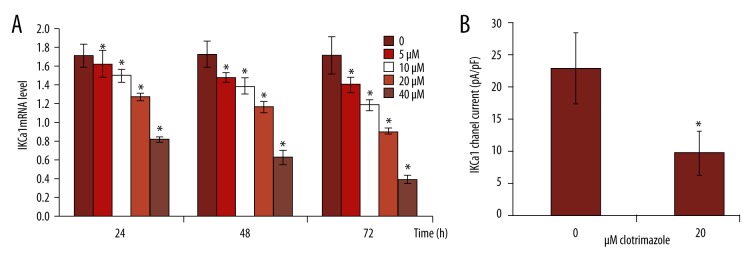
To determine whether IKCa1 expression contributes to cellular proliferation, we quantified the effect of an IKCa1 channel blocker, clotrimazole, on the proliferation of IKCa1cells using an MTT cell proliferation assay. We measured the effect of several different concentrations of clotrimazole – 0, 5, 10, 20, and 40 μM – on the number of HeLa cells. The number of HeLa cells was significantly reduced after treatment with clotrimazole at all concentration for 24, 48, and 72 h as compared to control cells (Figure 4A; p<0.05). Absorbance (A) gradually decreased along with the rate of cellular proliferation. Compared to control (ethanol only) cells, the rate of inhibition by clotrimazole increased as a function of concentration and length of incubation. This was evident by a decrease in cell proliferation compared to control groups (Figure 4B). In summary, the proliferation of HeLa cells can be inhibited by clotrimazole in a concentration- and time-dependent manner.
Figure 4.
The IKCa1 blocker clotrimazole reduces HeLa cell number and inhibits cellular proliferation compared to control. HeLa cell culture media was replaced with serum-containing media including 0, 5, 10, 20, and 40 μM clotrimazole inhibitors and grown for 24, 48, and 72 h. The number of cells were quantitatively assessed using a Coulter Counter (A). The rate of inhibition on cell proliferation was assessed using MTT assay (see methods) * P<0.05 compared with HeLa cell treated with 5 μM clotrimazole (B).
Clotrimazole decreases the expression level of IKCa1 mRNA and channel current
We observed a prominent downregulation in the average relative expression level of IKCa1 mRNA in HeLa cells with the increase of the clotrimazole concentration and the time of use compared to controls (Figure 5). The expression level of IKCa1 mRNA in HeLa cells treated by clotrimazole was significantly different at each time point (P<0.05) (Figure 5A).
Figure 5.
Expression of IKCa1 mRNA and channel current in HeLa cells are decreased by treatment with clotrimazole. (A) The average relative expression level of IKCa1 mRNA in HeLa cells treated by 0, 5, 10, 20, and 40 μM clotrimazole for 24, 48, and 72 h was detected by qRT-PCR. (B) IKCa1 channel current in HeLa cells treated with 0 or 20 μM clotrimazole was detected by patch clamp technique. All experiments were repeated 3 times with similar results. * Indicated significant difference (P<0.05) determined by t test.
We measured the current of IKCa1 channel by depolarizing voltage steps from a holding potential of −60 mv. IKCa1 channel current was 9.84±4.46 pA/Pf compared to 23.07±5.47 pA/pF in the controls when 20 μM clotrimazole was added to the bath solution. Statistical analysis showed that there was a significant difference between control and treatment groups (P<0.05) (Figure 5B).
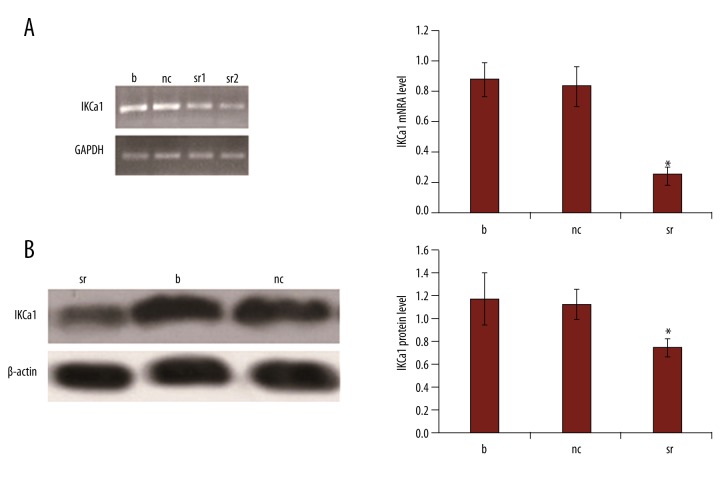
IKCal siRNAs decrease IKCal mRNA and protein expression
We used IKCal channel siRNA to inhibit the expression of IKCal channel in HeLa cells. At 48 h after transfection, the IKCal-specific siRNAs significantly downregulated IKCal mRNA expression levels by 70.24% (Figure 6A, p<0.05). Similarly, the expression level of IKCal protein in HeLa cells transfected with IKCal-specific siRNAs for 48 h was also significantly decreased by 33.93% (Figure 6B, p<0.05). There was no significant difference between the IKCal mRNA and protein expression of blank and negative controls (Figure 6, p>0.05).
Figure 6.
IKCa1 siRNAs downregulates IKCa1 mRNA and protein expression in HeLa cells. (A, B) Effect of the IKCa1-specific siRNAs on IKCa1 mRNA and protein expression levels, respectively. “nc” represents cells transfected with pGensil-HK+Lipo. “sr1” represents that HeLa cells were transfected with pGenesil-IKCa1siRNA+ Lipo and “sr2” represents a repeat of this experiment. The letter b (blank) represents cells were transfected with RPMI-1640+Lipo. At 48 h post-transfection, mRNA and protein levels were quantified and normalized to housekeeping genes GAPDH and β-actin, respectively. Data are the mean values ±SE of 3–5 independent transfections. * p<0.05, vs. non-transfected (blank) cells as determined by t test.
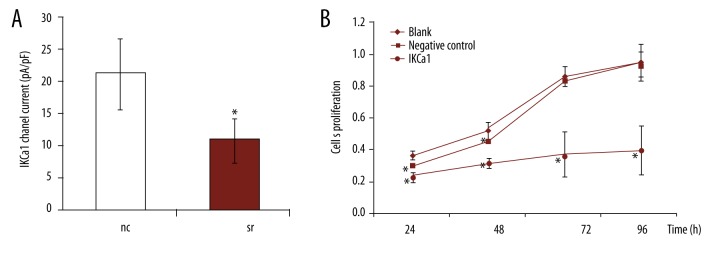
IKCa1 siRNAs inhibited IKCa1 channel activity and proliferation of HeLa cells
We used the IKCa1-specific siRNA to test for functional suppression of IKCa1 channels. As seen in Figure 7A, the IKCa1-specific siRNA induced a 48.69% decrease in the IKCa1 current density at 48 h after transfection (10.93±4.4 pA/pF for IKCa1siRNA vs. 21.3±5.51 pA/pF for negative control siRNA). The difference in IKCa1current density between IKCa1 siRNA and negative control siRNA was significant (p<0.05).
Figure 7.
IKCa1 gene silencing decreases K+ current densities and proliferation rates in HeLa cells. (A) Average IKCa1 currents were measured at 48 h after transfection with negative control siRNA (NC) or siRNA targeting IKCa1. Data are the mean values ±SE from 14–15 cells per experimental group (n=6. * p<0.05) vs. negative control and non-transfected (blank) cells. (B) Effect of transfection with negative control (NC) or IKCa1 siRNAs on proliferation of HeLa cells measured at 24, 48, 72, and 96 h post-transfection by MTT assay. Data are the mean values ±SE of 3 independent cell transfections. * p<0.05, vs. non-transfected (blank) cells.
As shown in Figure 7B, IKCa1 siRNA inhibited the proliferation of HeLa cells. The MTT proliferation assays showed that the value of absorbance (A) increased gradually with growth time in control cells, but cells transfected with IKCal siRNA proliferated at a slower rate. This reduction in growth in HeLa cells transfected with IKCa1 siRNA was statistically significant (p<0.05, Figure 7b). Cells transfected with an siRNA targeting an unrelated IKCa1channel grew in a manner similar to the blank (p>0.05).
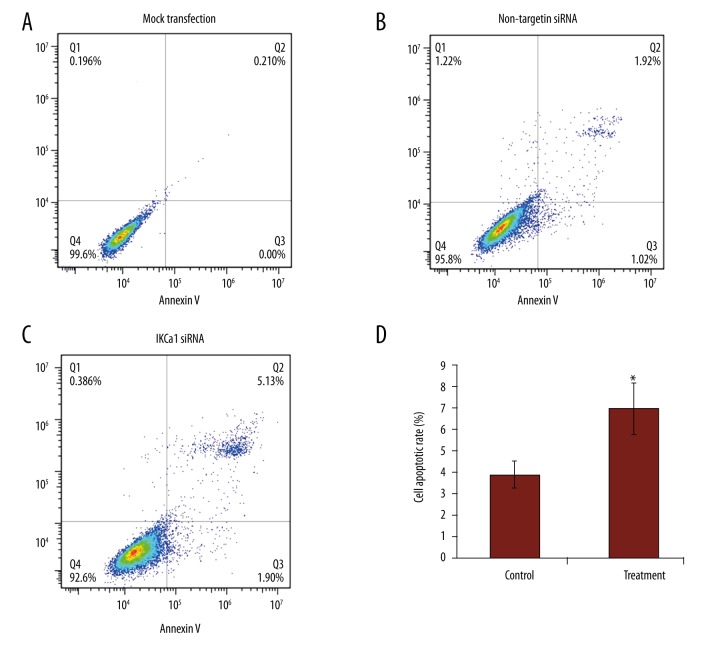
IKCa1 downregulation induces apoptosis of HeLa cells
The IKCal-specific siRNAs significantly downregulated IKCal mRNA expression levels (Figure 6A), which induced a significant increase in the proportion of apoptotic cells in HeLa cells (7.03±1.21% vs. 3.94±0.65%) (Figure 8). The percentages of HeLa cells undergoing early apoptosis and late apoptosis were increased (P<0.05). The percentage of cells undergoing apoptosis was determined by the sum of cells in early and late apoptosis.
Figure 8.
HeLa cells transfected with IKCal siRNAs induces apoptosis. HeLa cells were assayed by flow cytometry for annexin V binding and PI staining 72 h after infection. The logarithm of annexin V-fluorescein isothiocyanate fluorescence and the logarithm of PI fluorescence were plotted on the x and y axes of the cytogram, respectively. The lower right quadrant shows the percentage of viable cells in the early stages of apoptosis (i.e., annexin V-positive, PI-negative cells), the upper right quadrant shows the percentage of cells in a later stage of apoptosis or necrosis (annexin V-positive, PI-positive cells), the lower left shows the percentage of cells in the proliferating-senescence state, and the upper left quadrant shows the percentage of dead cells. (A) and (B) represent blank control and negative control cells infected with expressing control siRNA but not target IKCal, respectively. (C) Represents cells infected with IKCa1 siRNA. (D) Percentages of cells in apoptosis. The results are presented as the mean of 3 similar experiments. * P<0.05 compared with negative control.
Discussion
The incidence of cervical cancer has been gradually increasing, but the molecular mechanisms underlying the proliferation of cervical cancer are still unclear. Activation of Cl−, K+, aquaporin, and Ca2+ channels occur during cell proliferation [33–35]. Over the past 2 decades, these and other ion channels have been identified as important contributors to cell proliferation in normal and cancerous cells [36,37]. K+ channels are thought to be important for setting the membrane potential and act as the driving force for Ca2+ influx, and both mechanisms are considered crucial for volume regulation in growing cells [37].
Data from numerous cell types have also shown that potassium channels are essential for cell proliferation, and their dysregulation appears to play a role in carcinogenesis [15]. Ca2+-dependent potassium channels (KCa) also regulate cell cycle progression and are attracting increasing attention as a potential therapeutic target. Intermediate-conductance Ca2+-activated K+ channel, IKCa1(also called IK1, SK4, hSK4, hKCa4, and KCa3.1) is one of the KCa1 channels that regulates proliferation and migration of cancer cells [5,38,39]. IKCa1 primarily controls late G1 phase, G1/S transition, and G2/M phase. In breast, prostate, and endometrial cancers, IKCa1 regulates G1 and G1/S transition [5,10,15]. Recent important reports have suggested that IKCa1 channels were upregulated in several solid cancers such as prostate cancer, hepatocellular carcinoma, endometrial, mammary carcinoma, glioblastoma [11–13], several cancer cell lines [5,16], tumor vessels, proliferating endothelial cells [40,41], and activated T cells [42,43]. However, the effect of IKCa1 on the growth of cervical cancer cells was unknown.
Our study demonstrated that IKCa1 is upregulated in cervical cancer tissues and cells at the mRNA level and protein level (Figures 1–3). We found that IKCa1mRNA was expressed in 73.33% (22 out of 30) of cervical cancer tissues and in 11.11% (2 out of 18) of normal cervical tissues, which suggests that there is preferential expression of the IKCa1in cervical cancer tissues. Our immunohistochemical analysis revealed that IKCa1 protein was expressed in all 30 cervical cancer tissues, but was either absent or very low in control tissues. We also found that IKCa1 expression in cervical cancer tissues with different clinical features of disease was significantly correlated to histologic grade. Cervical cancer is classified as grades G1 through G3 based upon their histopathological characteristics, with higher grades being more de-differentiated and malignant [44].Grade G3 cervical cancers have a very high proliferative potential [44]. As shown in Figure 2 and Table 1, higher histological grade of cervical cancer tissue is associated with higher expression of IKCa1 protein. Our results suggest that the IKCa1 channel is involved in the promotion of cell proliferation in de-differentiated and malignant cervical cancer cells.
Our study also showed that the IKCa1 channel is expressed in HeLa cells (Figure 3). Our experiments show that the IKCa1 channel is critically involved in the calcium-induced hyperpolarization of the membrane potential of HeLa cells and regulation of cell growth. Our observations are in line with several other lines of evidence that have implicated IKCa1 channels in the regulation of growth rate in numerous types of malignant and nonmalignant mammalian cells [15,45–50]. Observations that the inhibitor of the IKCa1, clotrimazole, strongly reduced tumor load and metastases in vivo in several cancer types is important [51–53]. Our experimental results are in line with this effect of clotrimazole and we demonstrated this IKCa1 inhibitor significantly inhibited the growth of HeLa cells in a dose- and time-dependent manner (Figure 4A, 4B). Knockdown of IKCa1 using siRNA also significantly downregulated IKCal mRNA expression levels (Figure 6), which induced apoptosis of HeLa cells (Figure 8) and then inhibited the proliferation of HeLa cells (Figure 7). Many other reports indicated that IKCa1 channels regulated the proliferation of HeLa cells through regulating membrane potential [15,34,37,45,46]. In this study, we showed that clotrimazole and IKCa1-targeting siRNA can markedly decrease the IKCa1 channel current. A decline in IKCa1 channel current reduces the ability to influx Ca2+, which weakens the intracellular Ca2+ signal that regulates cell cycle progression and results in the suppressed proliferation in HeLa cells that we observed. The improved apoptosis of HeLa cells by downregulation of IKCal mRNA expression is another reason for the suppressed proliferation.
We showed that clotrimazole can downregulate IKCa1 gene expression in a dose- and time- dependent manner (Figure 5). Our results are consistent with several studies, and Susumu et al. [48] found that TRAM-34, a derivative of clotrimazole, also decreased IKCa1 gene expression. Tharp et al. [55] also found that pharmacological blockade of KCa3.1 prevents upregulation of KCa3.1. In addition to clotrimazole, there are several studies that have linked additional regulatory elements to the control of IKCa1. Regulatory elements such as the repressor element-1 silencing transcription factor (REST) and nuclear transcription factor activator protein-1, AP-1, (Fos/Jun) are transcriptional regulators of KCa3.1 [56–58]. There are reports that the IKCa1 promoter region contains an AP-1 binding site necessary for promoter activity [57,59]. There is also a report that downregulation of REST can increase expression of IKCa1 [56]. In addition, Tharp et al. [55] have shown that in proliferative, de-differentiated vascular smooth muscle cells, upregulation of KCa3.1 is followed by KCa3.1 promoter histone acetylation. Susumu et al. consider that TRAM-34 prevents KCa3.1 promoter activation at the epigenetic level [48]. Owens et al. showed that increased histone H4 acetylation (H4Ac) within the IKCa1 promoter region is associated with increased transcription factor binding required for promoter activity, which is a mechanism that has been proposed to maintain chromatin in an open state for easy access of transcription factors [60].
Together, these data suggest a model in which IKCa1-induced hyperpolarization would increase Ca2+ influx, which would in turn activate AP-1 and post-translational histone acetylation to further drive the expression of IKCa1 in a positive feedback manner. Therefore, our blockage of IKCa1 using clotrimazole decreased Ca2+ influx and resulted in the downregulation of IKCa1 mRNA expression. It is possible that the upregulation of REST mediated by clotrimazole may be another pathway of decreasing IKCa1 mRNA expression level. Further studies are required to elucidate the mechanism by which clotrimazole regulates IKCa1 mRNA expression.
Conclusions
The intermediate-conductance Ca2+-activated potassium channel is highly expressed in cervical cancer tissues, and its expression is significantly correlated to histologic grade. The IKCa1-blocker, clotrimazole, and IKCa1 gene silencing produced a significant reduction of proliferation as well as the IKCa1 channel current and an increase of apoptotic in HeLa cells. These experiments identified and confirmed IKCa1 as a new molecular marker of disease progression through its action on cell growth in cervical cancer tissues and HeLa cells.
Acknowledgments
We gratefully acknowledge Drs. Liyi W., Qianchuan R., Shuquan D., Yongping Z., and Zhaoming L. of the Department of Gynaecology and Obstetrics of the Affiliated Hospital of Southwest Medical University for their outstanding contributions in collecting clinical specimens.
Footnotes
Conflicts of interest
No potential conflicts of interest were disclosed.
Source of support: Departmental sources
References
- 1.Arbyn M, Castellsagué X, Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Hildesheim A, Berrington de González A. Etiology and prevention of cervical adenocarcinomas. J Natl Cancer Inst. 2006;98:292–293. doi: 10.1093/jnci/djj098. [DOI] [PubMed] [Google Scholar]
- 3.Szalmás A, Kónya J. Epigenetic alterations in cervical carcinogenesis. Seminars in Cancer Biology. 2011;19:144–52. doi: 10.1016/j.semcancer.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd MC, Duffy SM, Harris T, et al. KCa3.1 Ca2+ activated K+ channels regulate human airway smooth muscle proliferation. Am J Respir Cell Mol Biol. 2007;37:525–31. doi: 10.1165/rcmb.2006-0358OC. [DOI] [PubMed] [Google Scholar]
- 5.Ouadidahidouch H, Roudbaraki M, Delcourt P, et al. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004;287(1):C125–34. doi: 10.1152/ajpcell.00488.2003. [DOI] [PubMed] [Google Scholar]
- 6.Xia XM, Fakler B, Rivard A, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–7. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 7.Toyama K1, Wulff H, Chandy KG, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–37. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neylon CB, Lang RJ, Fu Y, et al. Molecular cloning and characterization of the intermediate-conductance Ca(2+)-activated K(+) channel in vascular smooth muscle: Relationship between K(Ca) channel diversity and smooth muscle cell function. Circ Res. 1999;85:e33–43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 9.Pedarzani P, Stocker M. Molecular and cellular basis of small – and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZH, Shen B, Yao HL, et al. Blockage of intermediate-conductance-Ca(2+)-activated K(+) channels inhibits progression of human endometrial cancer. Oncogene. 2007;26:5107–14. doi: 10.1038/sj.onc.1210308. [DOI] [PubMed] [Google Scholar]
- 11.Ruggieri P, Mangino G, Fioretti B, et al. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS One. 2012;7:e47825. doi: 10.1371/journal.pone.0047825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XW, Liu JW, Zhang RC, et al. Inhibitory effects of blockage of intermediate conductance Ca~(2+)-activated K~+ channels on proliferation of hepatocellular carcinoma cells. J Huazhong Univ Sci Technolog Med Sci. 2013;33:86–89. doi: 10.1007/s11596-013-1076-0. [DOI] [PubMed] [Google Scholar]
- 13.Turner KL, Honasoge A, Robert SM, et al. A proinvasive role for the Ca 2+ -activated K + channel KCa3.1 in malignant glioma. Glia. 2014;62:971–81. doi: 10.1002/glia.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haren N, Khorsi H, Faouzi M, et al. Intermediate conductance Ca2+ activated K+ channels are expressed and functional in breast adenocarcinomas: Correlation with tumour grade and metastasis status. Histol Histopathol. 2010;25:1247–55. doi: 10.14670/HH-25.1247. [DOI] [PubMed] [Google Scholar]
- 15.Lallet-Daher H, Roudbaraki M, Bavencoffe A, et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009;28:1792–806. doi: 10.1038/onc.2009.25. [DOI] [PubMed] [Google Scholar]
- 16.Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003;471:157–64. doi: 10.1016/s0014-2999(03)01825-9. [DOI] [PubMed] [Google Scholar]
- 17.Jäger H, Dreker T, Buck A, et al. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004;65:630–38. doi: 10.1124/mol.65.3.630. [DOI] [PubMed] [Google Scholar]
- 18.Wulff H, Kolskiandreaco A, Sankaranarayanan A, et al. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem. 2007;14:1437–57. doi: 10.2174/092986707780831186. [DOI] [PubMed] [Google Scholar]
- 19.Köhler R, Wulff H, Eichler I, et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108:1119–25. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- 20.Si H, Grgic I, Heyken WT, et al. Mitogenic modulation of Ca 2+-activated K+ channels in proliferating A7r5 vascular smooth muscle cells. Br J Pharmacol. 2006;148:909–17. doi: 10.1038/sj.bjp.0706793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores CA, Melvin JE, Figueroa CD, Sepúlveda FV. Abolition of Ca 2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca 2+-dependent K+ channel Kcnn4. J Physiol. 2007;583:705–17. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Eur J Physiol. 2010;459:969–76. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- 23.Mcferrin MB, Turner KL, Cuddapah VA, Sontheimer H. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am J Physiol Cell Physiol. 2012;303:1070–78. doi: 10.1152/ajpcell.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua X, Deuse T, Chen YJ, et al. The potassium channel KCa3.1 as new therapeutic target for the prevention of obliterative airway disease. Transplantation. 2013;95:285–92. doi: 10.1097/TP.0b013e318275a2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian X, Francis M, Köhler R, et al. Positive feedback regulation of agonist-stimulated endothelial Ca2+ dynamics by KCa3.1 channels in mouse mesenteric arteries. Arterioscler Thromb Vasc Biol. 2014;34(1):127–35. doi: 10.1161/ATVBAHA.113.302506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiefer H, Blume AJ, Kaback HR. Membrane potential changes during mitogenic stimulation of mouse spleen lymphocytes. Proc Natl Acad Sci USA. 1969;98:121–27. doi: 10.1073/pnas.77.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strobl JS, Wonderlin WF, Flynn DC. Mitogenic signal transduction in human breast cancer cells. Gen Pharmacol. 1995;26:1643–49. doi: 10.1016/0306-3623(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro G, Catalano M, Sciaccaluga M, et al. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013;4:e773. doi: 10.1038/cddis.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabjerg M, Olivan-Viguera A, Hansen LK, et al. High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival. PLoS One. 2015;10:e0122992. doi: 10.1371/journal.pone.0122992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori F, Oda N, Sakuragi M. New histopathological experimental model for benign prostatic hyperplasia: Stromal hyperplasia in rats. J Urol. 2009;181:890–98. doi: 10.1016/j.juro.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 31.Birner P, Schindl M, Obermair A, et al. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–96. [PubMed] [Google Scholar]
- 32.Hamill OP, Marty A, Neher E, et al. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 33.Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–92. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- 34.Lang F, Föller M, Lang K, et al. Chapter Eleven – Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007;428:209–25. doi: 10.1016/S0076-6879(07)28011-5. [DOI] [PubMed] [Google Scholar]
- 35.Lehen’Kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene. 2007;26:7380–85. doi: 10.1038/sj.onc.1210545. [DOI] [PubMed] [Google Scholar]
- 36.Ouadidahidouch H, Ahidouch A. K(+) channels and cell cycle progression in tumor cells. Front Physiol. 2013;4:220. doi: 10.3389/fphys.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunzelmann K. Ion channels and cancer. J Membr Biol. 2005;205:159–73. doi: 10.1007/s00232-005-0781-4. [DOI] [PubMed] [Google Scholar]
- 38.Faouzi M, Chopin V, Ahidouch A, Ouadid-Ahidouch H. Intermediate Ca2+-sensitive K+ channels are necessary for prolactin-induced proliferation in breast cancer cells. J Membr Biol. 2010;234:47–56. doi: 10.1007/s00232-010-9238-5. [DOI] [PubMed] [Google Scholar]
- 39.Catacuzzeno L, Fioretti B, Franciolini F. Expression and role of the intermediate-conductance calcium-activated potassium channel KCa3.1 in glioblastoma. J Signal Transduct. 2011;2012:421564. doi: 10.1155/2012/421564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grgic I, Eichler I, Heinau P, et al. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2005;25:704–9. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- 41.Köhler R, Degenhardt C, Kühn M, et al. Expression and function of endothelial Ca(2+)-activated K(+) channels in human mesenteric artery: A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- 42.Fanger CM, Rauer H, Neben AL, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. J Biol Chem. 2001;276:12249–56. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- 43.Chandy KG, Wulff H, Beeton C, et al. K + channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–89. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haybittle JL, Blamey RW, Elston CW, et al. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–66. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Zhao L, Ma W, et al. The blockage of KCa3.1 channel inhibited proliferation, migration and promoted apoptosis of human hepatocellular carcinoma cells. J Cancer. 2015;6:643–51. doi: 10.7150/jca.11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.So EC, Huang YM, Hsing CH, et al. Arecoline inhibits intermediate-conductance calcium-activated potassium channels in human glioblastoma cell lines. Eur J Pharmacol. 2015;758:177–87. doi: 10.1016/j.ejphar.2015.03.065. [DOI] [PubMed] [Google Scholar]
- 47.Schickling BM, Aykin-Burns N, Leslie KK, et al. An inhibitor of Kchannels modulates human endometrial tumor-initiating cells. Cancer Cell Int. 2011;11:25. doi: 10.1186/1475-2867-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohya S, Niwa S, Kojima Y, et al. Intermediate-conductance Ca2+-activated K+ channel, KCa3.1, as a novel therapeutic target for benign prostatic hyperplasia. J Pharmacol Exp Ther. 2011;338:528–36. doi: 10.1124/jpet.111.182782. [DOI] [PubMed] [Google Scholar]
- 49.Ohya S, Imaizumi Y. Intermediate-conductance Ca2+-activated K+ channel KCa3.1 and its related molecules in T-lymphocytes. Inflammation & Cell Signaling. 2014;1 doi: 10-14800/ics.14327. [Google Scholar]
- 50.Grgic I, Kiss E, Kaistha BP, et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci USA. 2009;106:14518–23. doi: 10.1073/pnas.0903458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benzaquen LR, Brugnara C, Byers HR, et al. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat Med. 1995;1:534–40. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- 52.Khalid M, Shibata S, Hiura T. Effects of clotrimazole on the growth, morphological characteristics, and cisplatin sensitivity of human glioblastoma cells in vitro. J Neurosurg. 2009;90:918–27. doi: 10.3171/jns.1999.90.5.0918. [DOI] [PubMed] [Google Scholar]
- 53.Khalid MH, Tokunaga Y, Caputy AJ, Walters E. Inhibition of tumor growth and prolonged survival of rats with intracranial gliomas following administration of clotrimazole. J Neurosurg. 2005;103:79–86. doi: 10.3171/jns.2005.103.1.0079. [DOI] [PubMed] [Google Scholar]
- 54.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–22. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tharp DL, Wamhoff BR, Wulff H, et al. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscl Thromb Vasc Biol. 2008;28:1084–89. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheong A, Bingham AJ, Jing L, et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol Cell. 2005;20:45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 57.Ghanshani S, Wulff H, Miller MJ, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. J Biol Chem. 2000;275:37137–49. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Ji AK, Joo KY, et al. Globotriaosylceramide leads to K(Ca)3.1 channel dysfunction: A new insight into endothelial dysfunction in Fabry disease. Cardiovasc Res. 2011;89:290–99. doi: 10.1093/cvr/cvq333. [DOI] [PubMed] [Google Scholar]
- 59.Saito T, Fujiwara Y, Fujiwara R, et al. Role of augmented expression of intermediate-conductance CA 2+ -Activated K + channels in postischaemic heart. Clin Exp Pharmacol Physiol. 2002;29:324–29. doi: 10.1046/j.1440-1681.2002.03652.x. [DOI] [PubMed] [Google Scholar]
- 60.Mcdonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]