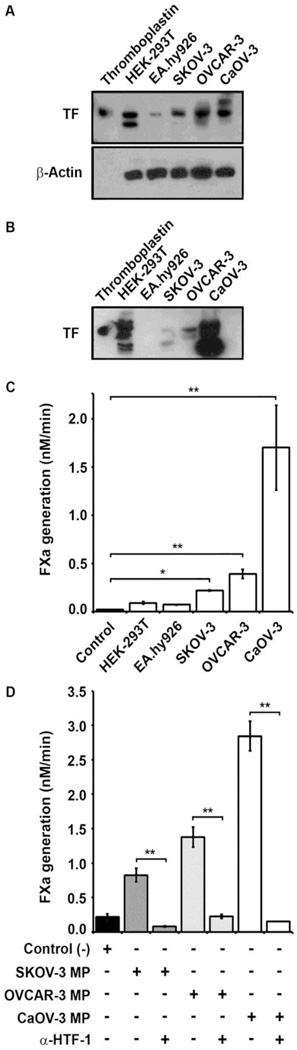
Figure 2. TF expression and MP-associated coagulant activity in epithelial ovarian cancer cell lines.
For Western blots whole cell lysates or MPs were prepared in lysis buffer containing 1% Triton-X 100, clarified by centrifugation and separated by 10% SDS-PAGE. (A–B) TF antigen was detected by immunoblotting (upper panel). (A) A placental-derived TF (Thromboplastin) standard (20ng) was loaded in lane 1 and cell lysates equivalent to 8×104 cells cells/lane were loaded in the remaining 5 lanes for HEK-293T (epithelial) and EA.hy926 (endothelial) cell lines; and human EOC cell lines SKOV-3, OVCAR-3 and CaOV-3. β-actin loading control is shown in the lower panel control. (B) MP fractions derived from each cell line were immunoblotted. Placental-derived TF (Thromboplastin) standard (20ng) was loaded in lane 1. TF coagulant activity of the MP fractions was determined by FX activation assay. (C) The rate of FXa generation (nM/min) by MP isolated from HEK-293T, EA.hy926, SKOV-3, OVCAR-3 and CaOV-3 cell lines is shown as the mean ± SD (n=3). Negative (buffer) control is shown on the left. (D) TF coagulant activity was inhibited by inhibitory anti-human TF antibody (α-HTF-1) versus IgG isotype control. The rate of FXa generation triggered by MP isolated from the human EOC cell lines in the absence or presence of α-HTF-1 (0.025mg/ml) is shown as the mean ± SD (n=3). Addition of α-HTF-1 reduced the rate of FXa generation to background levels for all three EOC cell lines, while the IgG isotype control (0.025mg/mL) had no significant effect. (C and D) Student’s t-test: * p<0.05, ** p<0.001).