Abstract
In the female rat, sexual receptivity (lordosis) can be facilitated by sequential activation of estrogen receptor (ER) α and G protein-coupled estrogen receptor 1 (GPER) by estradiol. In the estradiol benzoate (EB) primed ovariectomized (OVX) rat, EB initially binds to ERα in the plasma membrane that complexes with and transactivates metabotropic glutamate receptor 1a to activate β-endorphin neurons in the arcuate nucleus of the hypothalamus (ARH) that project to the medial preoptic nucleus (MPN). This activates MPN μ-opioid receptors (MOP), inhibiting lordosis. Infusion of non-esterified 17β-estradiol into the ARH rapidly reduces MPN MOP activation and facilitates lordosis via GPER. Tamoxifen (TAM) and ICI 182,780 (ICI) are selective estrogen receptor modulators that activate GPER. Therefore, we tested the hypothesis that TAM and ICI rapidly facilitate lordosis via activation of GPER in the ARH. Our first experiment demonstrated that injection of TAM intraperitoneal, or ICI into the lateral ventricle, deactivated MPN MOP and facilitated lordosis in EB-primed rats. We then tested whether TAM and ICI were acting rapidly through a GPER dependent pathway in the ARH. In EB-primed rats, ARH infusion of either TAM or ICI facilitated lordosis and reduced MPN MOP activation within 30 minutes compared to controls. These effects were blocked by pretreatment with the GPER antagonist, G15. Our findings demonstrate that TAM and ICI deactivate MPN MOP and facilitate lordosis in a GPER dependent manner. Thus, TAM and ICI may activate GPER in the CNS to produce estrogenic actions in neural circuits that modulate physiology and behavior.
Keywords: GPR30; GPER; G protein-coupled estrogen receptor 1; arcuate nucleus of the hypothalamus; Tamoxifen; ICI 182,780; lordosis
INTRODUCTION
The recent discoveries of novel estrogen receptors (ER), novel signaling pathways for classical ER’s, and the expression of multiple types of ER’s in a single neuron have illuminated the complexity of estrogen signaling. For example, the facilitation of sexual receptivity (lordosis) is governed by the actions of estradiol mediated over time by multiple types of ER initiated signaling pathways at multiple subcellular locations (reviewed in Micevych et al., 2015; Sinchak and Wagner, 2012). In ovariectomized (OVX) rats, sexual receptivity (lordosis) can be facilitated with either a single large dose of estradiol benzoate (EB; 5–50 μg), or by sequential treatment with a priming (2 μg EB) dose followed by non-esterified 17β-estradiol (E2) (Blaustein et al., 1987; Clemens and Weaver, 1985; Jones et al., 2013; Parsons et al., 1984; Quadagno et al., 1972; Sodersten and Eneroth, 1981).
In a model neurocircuit that regulates rat sexual receptivity that we study, steroid actions regulate the activity of proopiomelanocortin (POMC) neurons that originate in the arcuate nucleus of the hypothalamus (ARH) and project to the medial preoptic nucleus (MPN). The MPN is an important node in the neurocircuitry that regulates sexual receptivity (Acosta-Martinez and Etgen, 2002; Pfaus and Pfaff, 1992; Powers, 1972; Sinchak et al., 2007; Sinchak and Micevych, 2001; Sinchak et al., 2004; Sirinathsinghji, 1984; Torii et al., 1996, 1999). In this model circuit, EB initiates signaling via binding to a membrane-associated ERα (mERα) that complexes with, and transactivates metabotropic glutamate receptor-1a (mGluR1a; Dewing et al., 2007). This presumably excites, via neuropeptide Y-Y1 receptor, ARH POMC neurons that project to the MPN and release β-endorphin that activates and internalizes μ-opioid receptors (MOP) (Mills et al., 2004). This rapid and maintained EB-induced, mERα-mGluR1a-mediated MPN MOP activation appears to be actively inhibiting sexual receptivity, which is subsequently reduced for facilitation of lordosis locations (reviewed in Micevych et al., 2015; Sinchak and Wagner, 2012). For example, in EB-primed rats either steroid or pharmacological treatments that facilitate lordosis also reduce EB-induced MPN MOP activity (Dewing et al., 2007; Dewing et al., 2008; Eckersell et al., 1998; Long et al., 2014; Mills et al., 2004; Sanathara et al., 2011; Sinchak et al., 2013; Sinchak and Micevych, 2001). Conversely, in rats that are steroid-primed to induce sexual receptivity, infusion of MOP agonists in the MPN region rapidly and robustly inhibits lordosis (Acosta-Martinez and Etgen, 2002; Long et al., 2014; Pfaus and Pfaff, 1992; Sinchak et al., 2007; Sinchak and Micevych, 2001; Sirinathsinghji, 1984). Lesion studies and stimulation studies of the region also support this notion (Moss et al., 1974; Nance et al., 1977; Powers, 1972; Takeo, 1993). These inhibitory effects of the MPN may be regulating the lordosis output circuits downstream in the ventrolateral ventromedial nucleus of the hypothalamus and ventral tegmental area (Fahrbach et al., 1986; Takeo, 1993). Thus, activation of the MOP system in the MPN appears to prevent sexual receptivity until exposure to the proper levels and duration of steroid hormones.
In addition to the nuclear signaling of ER, the rapid initial actions of estradiol associated with MPN MOP activation are important for facilitation of lordosis. If the initial actions of estradiol are blocked by treating with a selective estrogen receptor modulator (SERM), tamoxifen (TAM), then sexual receptivity is not facilitated (Etgen and Shamamian, 1986). Likewise, if opioid activity in the region of the MPN is blocked at the time of estradiol priming, then subsequent facilitation of sexual receptivity does not occur (Torii et al., 1995, 1996, 1997, 1999). Finally, in our model system, if the initial mERα-mGluR1a signaling that rapidly activates MPN MOP is blocked by mGluR1a antagonists, the animals are nonreceptive (Dewing et al., 2007). These mERα-mGluR1a complexes are regulated by EB in a dose dependent manner. A 2 μg priming dose of EB that does not induce sexual receptivity maintains mERα-mGluR1a complexes in the plasma membrane and downstream MPN MOP activation for 48 hours (Mahavongtrakul et al., 2013). In contrast, a large 50 μg dose of EB that facilitates lordosis decreases mERα-mGluR1a complexes at 48 hours, suggesting that reducing mERα-mGluR1a signaling may facilitate lordosis and reduce MPN MOP activation (Mahavongtrakul et al., 2013). Therefore, we initially hypothesized blocking mERα-mGluR1a signaling reduces the excitatory input to β-endorphin neurons to deactivate MPN MOP and facilitate lordosis. Experiment 1 was designed to test the hypothesis that treatment with SERMs, TAM and ICI 182,780 (ICI), at 44 hours post-EB priming facilitates lordosis via reduction of mERα-mGluR1a signaling.
However, we recognized that TAM and ICI are also G protein-coupled estrogen receptor-1 (GPER; aka GPR-30 and GPER-1) agonists (Filardo et al., 2000; Filardo et al., 2002; Revankar et al., 2005; Vivacqua et al., 2006). Interestingly, in EB-primed rats, subsequent infusion of E2 into the third ventricle at the level of the ARH acts through a GPER dependent pathway that rapidly facilitates lordosis and reduces EB-induced MPN MOP activation (Long et al., 2014). Further, the timing of the EB + TAM/ICI treatments in experiment 1 was similar to that of the EB + E2 steroid paradigm that facilitates lordosis via GPER (Long et al., 2014). Therefore, in experiment 2 we tested the hypothesis that ARH GPER mediate ICI and TAM rapid facilitation of lordosis and deactivation of MPN MOP.
MATERIALS AND METHODS
Animals
Adult Long Evans OVX rats (200 to 225g; OVX by supplier) and male rats (200 to 225g; Charles River Laboratory Inc., Wilmington, MA) were housed in a light/climate controlled vivarium (12/12 L/D cycle, lights on at 0600h, 70° F) with food/water provided ad-libitum. Females were doubled housed in cages with pine bedding used in testing arena and single housed after cannulation surgery. Males were double housed in similar cages. All procedures were approved by the California State University, Long Beach IACUC.
Steroid priming
In all experiments EB was dissolved in safflower oil and delivered by subcutaneous (s.c.) injection (2 μg EB/0.1 ml oil). This mimics proestrus estradiol levels and alone does not induce sexual receptivity (Geary and Asarian, 1999; Micevych et al., 1996; Sanathara et al., 2011). Animals were cycled with 2 μg EB once every four days totaling 4 cycles (Long et al., 2014).
Experiment 1
We tested the hypothesis that TAM and ICI deactivate MPN MOP and facilitate lordosis in 2 μg EB-primed OVX rats. The ICI treated rats were implanted with a unilateral guide canula aimed at the lateral ventricle (LV). All animals were primed with EB and 44 hours later TAM animals received an intraperitoneal injection of either TAM (10 mg/kg/0.5 ml; Tocris Bioscience) or DMSO, whereas, ICI treated rats received a LV infusion of either ICI (1.98 nmol/1.0 μl; Tocris Bioscience), or DMSO (1 μl; Sigma-Aldrich). Four hours later rats were tested for sexual receptivity. On the next EB cycle, treatments were replicated and 4 hours after drug treatment rats were perfused with chilled 0.9% saline, followed by chilled 4% paraformaldehyde and brains were processed for MOP immunohistochemistry (Long et al., 2014; Sanathara et al., 2011).
Experiment 2
We tested whether TAM and ICI rapidly facilitate sexual receptivity and deactivate MPN MOP via activation of GPER in the ARH. OVX rats were primed with 2 μg EB, and received bilateral ARH drug infusions (0.5 μl volume per side) at 47.25, and 47.5 hours post-EB injection. Animals were first infused with either GPER antagonist, G-15, (35 nmol per side; Sigma-Aldrich) or DMSO. Fifteen minutes later, each rat received a final infusion of either TAM (0.27 nmol/side), ICI (0.99 nmol/side), or DMSO control. Animals were then tested for sexual receptivity thirty minutes later. On the next EB cycle animals received the same drug treatments, were perfused, and brains processed for MOP immunohistochemistry.
Stereotaxic surgery
All animals were anesthetized using 2–3% isoflurane and injected with an analgesic (Rimadyl, s.c. 5mg/kg; Western Medical Supply, Arcadia, CA). In experiment 1, stainless steel unilateral cannulae (Plastics One, Roanoke, VA) were implanted using standard stereotaxic surgical procedures (Sanathara et al., 2011). Cannulae were directed at the LV using coordinates modified from (Paxinos and Watson, 2007); tooth bar = −3.3; coordinates (mm) from bregma: AP = −1.0; L = −1.4; V = −3.5 from dura.
In experiment 2, bilateral stainless steel guide cannulae (Plastics one) were surgically implanted into the ARH (coordinates from bregma; anterior −2.3mm, lateral +/− 0.5mm, ventral −6.8mm from dura; tooth bar set at −3.3; modified from (Paxinos and Watson, 2007; Sanathara et al., 2011). Animals were allowed to recover from surgery a week prior to behavioral testing.
Drug infusions
Drugs were infused using a Hamilton 25 μl syringe at the rate of 1 μl/min (Exp 1) or 0.5 μl/min (Exp 2) driven by an infusion pump (Stoelting Co., Wood Dale, IL; Sanathara et al., 2011).
Sexual receptivity testing
In experiment 1, behavior experiments began 4 hours after final drug treatment (48h post-EB). In experiment 2 each animal was tested 30 minutes after their final ARH infusion (48h post-EB). The female rat was placed into a Plexiglas testing arena, and a trained male rat was allowed to mount the female 10 times. The number of times the female displayed lordosis out of 10 mounts was recorded. Lordosis quotient (LQ) was calculated to measure sexual receptivity by dividing the number of lordosis responses by the total number of mounts and multiplied by 100 (Long et al., 2014; Sanathara et al., 2011). In experiment 1, LQ data underwent square root transformation and analysis via t-test, and for experiment 2 data were analyzed with a 1-way ANOVA and Holm-Sidak test (Sigma Stat V3.5).
Tissue collection and cannula guide placement confirmation
Brains were cryosectioned at 20 μm thickness, collected in wells containing phosphate buffered saline, (pH 7.5) and stored at 4°C until mounted for cannula placement confirmation or processed for immunohistochemistry. To confirm guide cannulae placement, sections through the ARH were mounted onto Superfrost Plus slides, thionin stained, and analyzed via bright field microscopy (Sanathara et al., 2011).
MOP immunohistochemistry and image analysis
In both experiments, the effects of TAM and ICI on MPN MOP activation were measured by MOP fluorescent immunoreactivity (MOPi) intensity levels. MOP immunohistochemistry was performed on free-floating sections through the MPN as previously described (Dewing et al., 2007; Sanathara et al., 2011). Briefly, every fourth MPN section was incubated in MOP primary antibody (1:5000; Neuromics Antibodies Edina, MN; Dewing et al., 2007; Mills et al., 2004) and visualized using Fluorescein (FITC) staining associated with a Tyramide Signal Amplification kit (Perkin Elmer/Life Science Products, Boston, MA. MPN MOP internalization into early endosomes is positively correlated with an increase of MPN MOPi and is used to measure MPN MOP activation levels (Eckersell et al., 1998). MOPi intensity levels were estimated using epifluorescent photomicrographs taken from the dorsal region of the MPN at the level of the MPN centralis and quantifying the area of immuno-positive MPN MOP fiber density (Long et al., 2014). The greyscale images were adjusted for brightness and contrast using Adobe Photoshop (version 7.0; Adobe Systems Inc., San Jose, CA) by persons blind to treatment groups. MPN MOPi intensity levels were calculated in arbitrary units (AU) using ImageJ software (version 1.32j; National Institutes of Health, Bethesda, MD; Long et al., 2014; Sanathara et al., 2011). Levels of MPN MOPi intensity were analyzed via t-test (experiment 1), and 1-way ANOVA followed by post hoc Holm-Sidak test (experiment 2). For all experiments, effect size estimates were calculated by eta squared for ANOVAs and Cohen’s d for pair-wise comparisons.
RESULTS/DISCUSSION
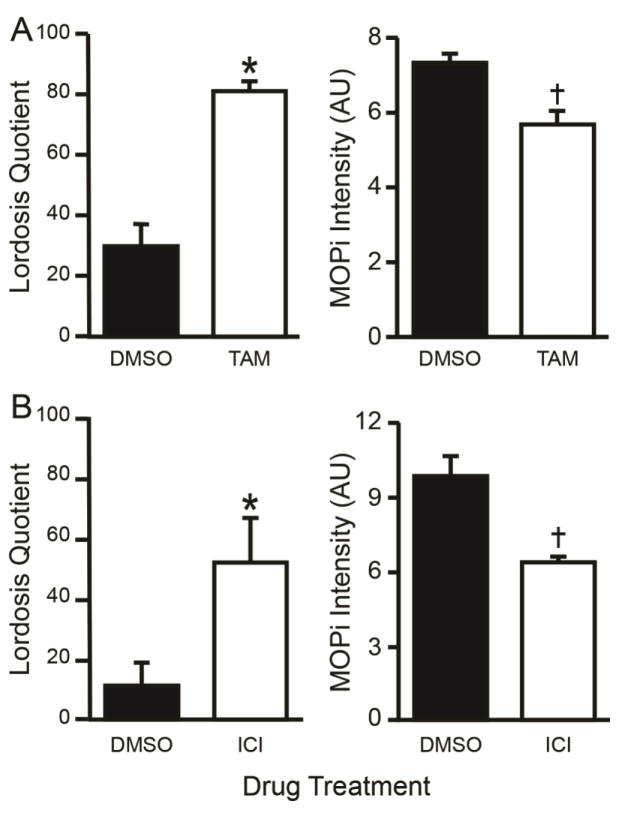
In experiment 1, treating 2 μg EB-primed rats with either TAM (intraperitoneal) or ICI (LV) facilitated sexual receptivity as measured by an increase in LQ compared to DMSO controls (TAM: t-test, df = 7, t = 5.066, P < 0.001; Cohen’s d: d = 3.83, r = 0.89; ICI: t-test, df = 6; t = 2.551, P = 0.043; Cohen’s d: d = 2.08, r = 0.72; Fig. 1A). Both the TAM and ICI treatments reduced MPN MOP activation levels as measured by MOPi compared to DMSO treated control rats (TAM: t-test, df = 7; t = 3.022, P = 0.019; Cohen’s d: d = 2.1, r = 0.73; ICI 182,780: t-test, df = 6, t = 2.650, P = 0.038; Cohen’s d: d = 2.16, r = 0.73; Fig. 1B). The TAM- and ICI-induced reduction of MPN MOP activation and facilitation of lordosis occurs presumably in response to a decrease in ARH β-endorphin neurotransmission to the MPN (Sinchak and Wagner, 2012). These data are consistent with our working model that TAM and ICI are acting through the ARH-MPN lordosis circuit, since MPN MOP activation was significantly reduced as seen in previous studies (Long et al., 2014; Sinchak and Wagner, 2012).
Fig 1.
Tamoxifen (TAM), and ICI 182,780 (ICI) facilitated sexual receptivity, as measured by lordosis quotient (LQ), and reduced MPN MOP activation as measured by MOP immunoreactivity (MOPi) intensity levels. OVX rats were primed with 2 μg EB (sc) and treated 44 h later with either A) TAM or DMSO (ip) or B) lateral ventricle infusions of ICI or DMSO, 4 h later they were tested for sexual receptivity. Four days after the EB priming for behavior, rats were given the same EB priming followed by the same drug treatments and were perfused followed by MOP immunohistochemistry. The TAM and ICI treatments both facilitated lordosis and reduced MOPi staining intensity levels compared to the DMSO treated rats. ✱ = Significantly greater than DMSO (P < 0.001). † = Significantly less than DMSO (P < 0.001).
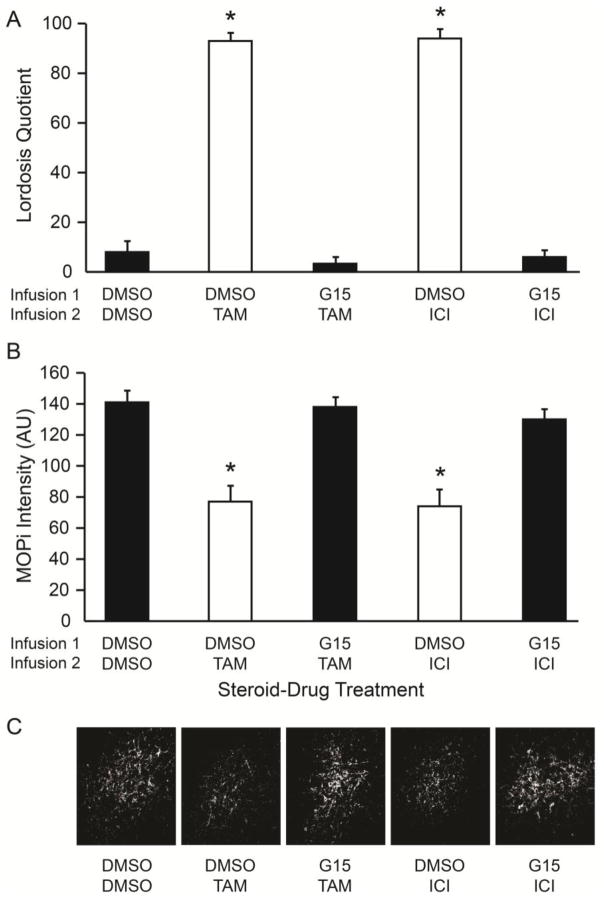
In experiment 2, we tested whether TAM and ICI rapidly signal through GPER in the ARH to deactivate MPN MOP and facilitate lordosis as observed with non-esterified 17β-estradiol (Long et al., 2014). LQ was significantly increased 30 minutes after bilateral ARH infusion of either DMSO-TAM (1-way ANOVA, df = 4, 23 F = 116, P < 0.001; eta squared = 0.95; Holm-Sidak, P < 0.001; Cohen’s d: d = 7.4, r = 0.97; Fig. 2A) or DMSO-ICI (Holm-Sidak, P < 0.001; Cohen’s d: d = 8.9, r = 0.98; Fig. 2A), compared to DMSO-DMSO controls. When rats received an ARH infusion of G15 prior to TAM or ICI, the LQ was significantly reduced (Holm-Sidak, P < 0.001; DMSO-TAM v G15-TAM - Cohen’s d: d = 7.4, r = 0.97; DMSO-ICI v G15-ICI - Cohen’s d: d = 7.7, r = 0.97; Fig 2A) and equivalent to the DMSO-DMSO infused rats (Holm-Sidak, P > 0.05; DMSO-DMSO v G15-TAM - Cohen’s d: d = 0.0, r = 0.0; DMSO-DMSO v G15-ICI - Cohen’s d: d = 0.37, r = 0.18; Fig 2A). The amount of MPN MOPi was significantly reduced in both the DMSO-TAM, (1-way ANOVA, df = 4, 23, F = 99, P < 0.001; eta squared = 0.95; Holm-Sidak, P < 0.001; Cohen’s d: d = 7.2, r = 0.96; Fig. 2B) and DMSO-ICI (Holm-Sidak, P < 0.001; Cohen’s d: d = 7.5, r = 0.97; Fig. 2B) treatment groups, as compared to DMSO-DMSO controls. Administration of G15 prior to TAM or ICI blocked their effects producing MOPi levels that were significantly higher than DMSO-TAM and DMSO-ICI (Holm-Sidak, P < 0.001; DMSO-TAM v G15-TAM - Cohen’s d: d = 8.0, r = 0.97; DMSO-ICI v G15-ICI - Cohen’s d: d = 7.2, r = 0.96; Fig 2B), and equivalent to DMSO-DMSO controls (Holm-Sidak, P > 0.05; DMSO-DMSO v G15-TAM - Cohen’s d: d = 0.42, r = 0.21; DMSO-DMSO v G15-ICI - Cohen’s d: d = 1.52, r = 0.61; Fig. 2B). Thus, direct exposure of TAM or ICI to the ARH results in the rapid (within 30 minutes) deactivation of MPN MOP and facilitation of lordosis. Further, these data indicate that GPER signaling plays an essential role in the TAM/ICI facilitation of lordosis, since the selective blockade of ARH GPER inhibited TAM and ICI facilitative actions. It is unlikely that there are residual effects of the SERMs on the MPN MOPi intensity levels from the behavioral experiment. If ICI/TAM were still active, the SERMs would have blocked the ERα-mGluR1a-induced increase in MPN MOPi intensity levels in the G15/ICI and G15/TAM treated animals. Further, the relatively small amounts of drugs infused, and in the rat the half-life of TAM is approximately 21 hours (Choi and Kang, 2008) and ICI is 4.3 to 18.5 hours in rat should have either cleared or reduced the concentrations to be ineffective (Robertson and Harrison, 2004; Rosario, 2002).
Fig 2.
Tamoxifen (TAM) and ICI 182,780 (ICI) rapid facilitation of lordosis and deactivation of MPN MOP was blocked by inhibition of ARH GPER. OVX rats primed with 2 μg EB received infusions into the ARH sequentially at 47.25 (Infusion 1), and 47.5 h (Infusion 2) post EB. Thirty minutes after the Infusion 2, each rat was tested for sexual receptivity as measured by lordosis quotient (LQ). On the next EB cycle, animals were given the same treatments and brains collected to measure the relative MPN MOP activation levels using MOP immunoreactivity (MOPi) staining intensity levels in arbitrary units (AU). A) TAM and ICI infused rats had a significantly increased LQ compared to DMSO controls, and administration of GPER antagonist, G15, prior to TAM or ICI significantly reduced the LQ compared to TAM or ICI only treated rats. B) Infusion of TAM and ICI significantly reduced MPN MOPi intensity levels compared to DMSO controls, and pretreatment with G15 prior to TAM or ICI blocked the reduction observed with TAM or ICI only treatment. C) Photomicrographs illustrating examples of MPN MOPi fiber densities for each treatment group used for MOPi intensity measures after contrast and brightness manipulation. ✱ = significantly different than other groups (p < 0.001).
In the context of our model circuit, these experiments indicate that TAM and ICI act via a GPER dependent signaling pathway originating in the ARH that reduces β-endorphin neurotransmission to MPN MOP (Sinchak and Wagner, 2012). TAM and ICI likely act through the same ARH GPER pathway that mediates E2 facilitation of lordosis and deactivation of MPN MOP (Long et al., 2014). One intriguing possibility is that TAM and ICI are activating ARH plasma membrane GPER that directly regulate orphanin FQ/nociception (OFQ) neurons to reduce β-endorphin activity via activation of opioid receptor like receptor-1 (ORL-1) to facilitate lordosis (Chokr et al., 2016; Feri et al., 2016; Long et al., 2015; Tran et al., 2015). It is unclear exactly how TAM and ICI are interacting with GPER to facilitate lordosis, and what signaling pathway(s) is activated to alter neurotransmission to facilitate lordosis (Prossnitz and Barton, 2014; Srivastava and Evans, 2013). Our initial hypothesis that disrupting ERα-mGluR1a signaling facilitates lordosis was not supported. If reducing only ERα-mGluR1a were sufficient to facilitate lordosis, then the G15/TAM and G15/ICI animals should have been sexually receptive. Further, in the Long et al., 2015 study, ARH E2 infusions that facilitated lordosis would presumably activate ERα-mGluR1a and GPER simultaneously, indicating the inhibitory effects induced by GPER activation are greater than the ERα-mGluR1a excitatory effects. Thus, TAM/ICI inhibition of ERα-mGluR1a signaling may augment GPER actions, but is not sufficient to facilitate lordosis and requires inhibitory input to the ARH β-endorphin neurons (Mahavongtrakul et al., 2013). Thus, TAM and ICI actions mediated by GPER appear to increase inhibitory input to ARH β-endorphin neurons that project to the MPN, which may be augmented by blocking ERα-mGluR1a excitation. Present experiments also demonstrate that peripherally administered TAM can have effects in the CNS.
CONCLUSIONS
The SERMs TAM and ICI are therapeutics for estrogen responsive cancers. They inhibit the activity of ERα & β, but have estrogenic actions via the activation of GPER. Presently, we provide evidence in vivo that TAM and ICI activate a subpopulation of GPER in the ARH to rapidly deactivate MPN MOP and facilitate lordosis in 2 μg EB primed rats. TAM and ICI infused directly into the ARH facilitated lordosis and deactivated MPN MOP, as seen with E2 activation of GPER (Long et al., 2014). Further, ARH infusions of a GPER antagonist, G15, prior to TAM or ICI treatment blocked both the facilitation of lordosis, and MPN MOP deactivation. Although inhibition of mERα-mGluR1a via TAM/ICI may also contribute the facilitation of lordosis and deactivation of MPN MOP, it is likely that TAM, ICI, and E2 are all acting via the same GPER dependent signaling mechanisms. We also demonstrate that peripheral injection of TAM can modulate hypothalamic circuits and alter sexual behavior. Thus, these results support that effects of TAM and ICI therapy may be produced by either inhibition of classical ER, or activation of GPER.
HIGHLIGHTS.
Tamoxifen (TAM) and ICI 182,780 (ICI) were infused into the hypothalamic arcuate nucleus (ARH)
TAM or ICI facilitated lordosis in 30 minutes in estradiol primed female rats
ARH G protein-coupled estrogen receptor 1 (GPER) mediated the TAM/ICI actions
ARH GPER activation deactivated medial preoptic nucleus μ-opioid receptors
Peripheral TAM can modulate hypothalamic circuits to affect behavior
Acknowledgments
Authors thank Nayna Sanathara, Yvette Yonan, Shrey Kanjiya, Kim Nguyen, Steven Nguyen, Martin Vignovich, Timbora Chuon, Amaleena Chhan, Daniel Tran, and Brad Huss for their technical assistance. Research was supported by NIH grant R01HD058638 NICHD (KS); 5R25GM071638 & GM07163 Research Initiative for Science Enhancement (RISE – CSULB); Doris A. Howell Foundation - CSUPERB Research Scholar Award (SC) and NIH Building Infrastructure Leading to Diversity (BUILD) 8UL1GM118979-02; 8TL4GM118980-02; 8RL5GM118979-02 (CSULB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Acosta-Martinez M, Etgen AM. Activation of mu-opioid receptors inhibits lordosis behavior in estrogen and progesterone-primed female rats. Horm Behav. 2002;41:88–100. doi: 10.1006/hbeh.2001.1741. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Finkbohner R, Delville Y. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinology. 1987;45:152–159. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kang KW. Enhanced tamoxifen bioavailability after oral administration of tamoxifen in rats pretreated with naringin. Arch Pharm Res. 2008;31:1631–1636. doi: 10.1007/s12272-001-2161-7. [DOI] [PubMed] [Google Scholar]
- Chokr SM, Long N, Chhan A, Sinchak K. Tamoxifen and ICI 182, 780 activation of G protein-coupled estrogen receptor 1 (GPER) acts through orphanin FQ (nociception) neurons to rapidly facilitate sexual receptivity, Society for Neuroscience. Neuroscience Meeting Planner; Washington, DC, San Diego, CA. USA. 2016. No.252.04. [Google Scholar]
- Clemens LG, Weaver DR. The role of gonadal hormone in the activation of feminine sexual behavior. In: Adler N, Pfaff D, Goy RW, editors. Handbook of Behavioral Neurobiology. Plenum Press; New York: 1985. pp. 183–227. [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinol. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen AM, Shamamian P. Regulation of estrogen-stimulated lordosis behavior and hypothalamic progestin receptor induction by antiestrogens in female rats. Hormones and Behavior. 1986;20:166–180. doi: 10.1016/0018-506x(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. J Comp Neurol. 1986;247:364–382. doi: 10.1002/cne.902470307. [DOI] [PubMed] [Google Scholar]
- Feri M, Phan J, Sinchak K. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2016. G protein-coupled estrogen receptor 1 (GPER) is found in the plasma membrane and cytoplasmic fractions of tissue from the arcuate nucleus of the hypothalamus, Society for Neuroscience. San Diego, CA USA No.252.201. [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67:141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Jones SL, Farrell S, Gregory JG, Pfaus JG. Sensitization of sexual behavior in ovariectomized rats by chronic estradiol treatment. Hormones and behavior. 2013 May 4; doi: 10.1016/j.yhbeh.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Long N, Serey C, Sinchak K. 17beta-estradiol rapidly facilitates lordosis through G protein-coupled estrogen receptor 1 (GPER) via deactivation of medial preoptic nucleus mu-opioid receptors in estradiol primed female rats. Horm Behav. 2014;66:663–666. doi: 10.1016/j.yhbeh.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NP, Chokr SM, Sinchak K. Estradiol-GPER facilitation of lordosis in the female rat is via direct regulation of the orphanin FQ-ORL-1 system in the arcuate nucleus, Neuroscience Meeting Planner. Vol. 2015. Washington, DC: Society for Neuroscience; 2015. Chicago, Il Program No. 715.713. [Google Scholar]
- Mahavongtrakul M, Kanjiya MP, Maciel M, Kanjiya S, Sinchak K. Estradiol dose-dependent regulation of membrane estrogen receptor-alpha, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinol. 2013;154:3251–3260. doi: 10.1210/en.2013-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Eckersell CB, Holland KL, Smith A. Induction of CCK mRNA levels in the limbic-hypothalamic circuit: Time course and site-specific effects of estrogen. J Neurobiol. 1996;30:465–479. doi: 10.1002/(SICI)1097-4695(199608)30:4<465::AID-NEU3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Wong AM, Mittelman-Smith MA. Estradiol Membrane-Initiated Signaling and Female Reproduction. Compr Physiol. 2015;5:1211–1222. doi: 10.1002/cphy.c140056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL, Paloutzian RF, Law OT. Electrical stimulation of forebrain structures and its effect on copulatory as well as stimulus-bound behavior in ovariectomized hormone-primed rats. Physiol Behav. 1974;12:997–1004. doi: 10.1016/0031-9384(74)90147-4. [DOI] [PubMed] [Google Scholar]
- Nance DM, Christensen LW, Shryne JE, Gorski RA. Modifications in gonadotropin control and reproductive behavior in the female rat by hypothalamic and preoptic lesions. Brain Res Bull. 1977;2:307–312. doi: 10.1016/0361-9230(77)90087-9. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, Snyder L, McEwen BS. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinology. 1984;39:25–30. doi: 10.1159/000123950. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; San Diego: 2007. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Pfaff DW. Mu-, delta-, and kappa-opioid receptor agonists selectively modulate sexual behaviors in the female rat: differential dependence on progesterone. Horm Behav. 1992;26:457–473. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]
- Powers BA, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175:1003–1005. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389:71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadagno DM, McCullough J, Langan R. The effect of varying amounts of exogenous estradiol benzoate on estrous behavior in the rat. Horm Behav. 1972;3:175–179. doi: 10.1016/0018-506x(72)90029-3. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Robertson JF, Harrison M. Fulvestrant: pharmacokinetics and pharmacology. Br J Cancer. 2004;90(Suppl 1):S7–10. doi: 10.1038/sj.bjc.6601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario LA. Pharmacology/Toxicology Review of NDA 21-344. Center for Drug Evaluation and Research; 2002. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-344_Fulvestrant_pharmr_P1.pdf. [Google Scholar]
- Sanathara NM, Moraes J, Kanjiya S, Sinchak K. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic nucleus mu-opioid receptors. Horm Behav. 2011;60:540–548. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Ponce L, Gomez L, Christensen A, Berger M, Micevych P. Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: A role for GABAB receptors. Horm Behav. 2013;64:136–143. doi: 10.1016/j.yhbeh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Eckersell CB, Micevych PE. Medial preoptic area delta-opioid receptors inhibit lordosis. Behav Brain Res. 2004;155:301–306. doi: 10.1016/j.bbr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirinathsinghji DJ. Modulation of lordosis behavior of female rats by naloxone, beta-endorphin and its antiserum in the mesencephalic central gray: possible mediation via GnRH. Neuroendocrinology. 1984;39:222–230. doi: 10.1159/000123983. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Eneroth P. Serum levels of oestradiol-17 beta and progesterone in relation to receptivity in intact and ovariectomized rats. J Endocrinol. 1981;89:45–54. doi: 10.1677/joe.0.0890045. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013;25:1219–1230. doi: 10.1111/jne.12071. [DOI] [PubMed] [Google Scholar]
- Takeo TCY, Sakuma Y. Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiology and Behavior. 1993;53:831–838. doi: 10.1016/0031-9384(93)90258-h. [DOI] [PubMed] [Google Scholar]
- Torii M, Kubo K, Sasaki T. Naloxone and initial estrogen action to induce lordosis in ovariectomized rats: the effect of a cut between the septum and preoptic area. Neurosci Lett. 1995;195:167–170. doi: 10.1016/0304-3940(95)11809-b. [DOI] [PubMed] [Google Scholar]
- Torii M, Kubo K, Sasaki T. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212:68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- Torii M, Kubo K, Sasaki T. Differential effects of beta-endorphin and Met- and Leu-enkephalin on steroid hormone-induced lordosis in ovariectomized female rats. Pharmacol Biochem Behav. 1997;58:837–842. doi: 10.1016/s0091-3057(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Torii M, Kubo K, Sasaki T. Facilitatory and inhibitory effects of beta-endorphin on lordosis in female rats: relation to time of administration. Horm Behav. 1999;35:271–278. doi: 10.1006/hbeh.1999.1526. [DOI] [PubMed] [Google Scholar]
- Tran DN, Long N, Serey C, Sinchak K. Abstract Viewer/Itinerary Planner. Vol. 2015. Washington, DC: Society for Neuroscience; 2015. Estradiol-G protein-coupled estrogen receptor 1 facilitation of sexual receptivity via direct regulation of the orphanin FQ/N-ORL-1 system in the arcuate nucleus of the hypothalamus, Society for Neuroscience. Chicago, IL. USA 715.711. [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol. 2006;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]