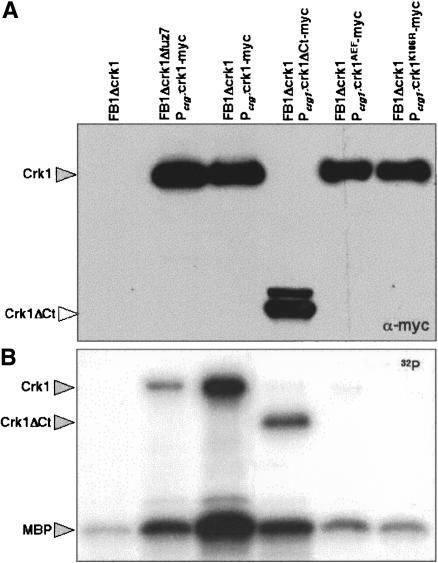
Figure 3.
Catalytic activity of Crk1 and derivates. Myc-tagged proteins were immunoprecipitated from cells extracts prepared from UMP12 (FB1 Δcrk1), UME63 (FB1 Δcrk1 Pcrg1:crk1-myc Δfuz7), UME61 (FB1 Δcrk1 Pcrg:crk1-myc), UME66 (FB1 Δcrk1 Pcrg:crk1ΔCt-myc), UME62 (FB1 Δcrk1 Pcrg:crk1AEF-myc), and UME69 (FB1 Δcrk1 Pcrg:crkK106R-myc) cells grown to OD600 of 0.5 in CMA. Protein kinase activity was measured by incubation of immunoprecipitates with purified Myelin Basic Protein (MBP) as substrate and [γ-32P]ATP. (Top) An 8% SDS-PAGE and immunoblot with anti-myc was used to show comparable levels of Crk1 proteins in the reaction mixtures. (Bottom) A 12.5% SDS-PAGE and autoradiography was used to detect in vitro phosphorylated MBP. We also found bands corresponding to autophosphorylated Crk1 and derivates.