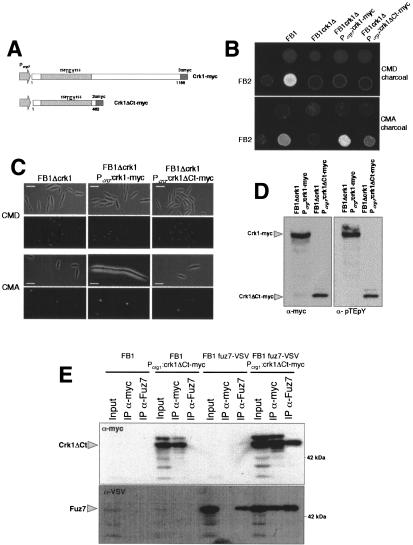
Figure 5.
The C-terminal domain of Crk1 is required for in vivo activity. (A) Scheme of the crk1 and the crk1ΔCt alleles fused to myc epitope under the control of Pcrg1. (B) The C-terminal domain is partially dispensable for mating. FB1, UMP12 (FB1 Δcrk1), UME61 (FB1 Δcrk1 Pcrg:crk1-myc), and UME66 (FB1 Δcrk1 Pcrg:crk1ΔCt-myc) cells were cospotted with FB2 cells in charcoal-complete medium plates with glucose (CMD charcoal) or arabinose (CMA charcoal) as carbon source. (C) The C-terminal domain is required for Crk1-mediated hyperpolarized growth. UMP12 (FB1 Δcrk1), UME61 (FB1 Δcrk1 Pcrg:crk1-myc), and UME66 (FB1 Δcrk1 Pcrg:crk1ΔCtmyc) cells were incubated in CMD and CMA for 6 h. Bar, 10 μm. (D) The C-terminal domain of Crk1 does not affect the T-loop phosphorylation. Extracts were prepared from UME61 (FB1 Δcrk1 Pcrg:crk1-myc) UME66 (FB1 Δcrk1 Pcrg:crk1ΔCt-myc) cells and analyzed as described in Figure 2. (E) Crk1ΔCt and Fuz7 coprecipitate. Cultures of FB1, UME66 (FB1 Δcrk1 Pcrg:crk1ΔCt-myc), UMS19 (FB1 fuz7-VSV), and UMS21 (FB1 fuz7-VSV Pcrg1:crk1ΔCt-myc) strains grown in CMA until OD600 of 0.5 were used to prepare whole-cell extracts (WCE). Immunoprecipitants obtained with anti-myc and anti-Fuz7 were analyzed by 8% SDS-PAGE, followed by immunoblotting with anti-VSV-peroxidase and anti-myc-peroxidase antibodies.