Abstract
Study Objectives:
Both depression and sleep complaints are very prevalent among kidney transplant (kTx) recipients. However, details of the complex relationship between sleep and depression in this population are not well documented. Thus, we investigated the association between depressive symptoms and sleep macrostructure parameters among prevalent kTx recipients.
Methods:
Ninety-five kTx recipients participated in the study (54 males, mean ± standard devation age 51 ± 13 years, body mass index 26 ± 4 kg/m2, estimated glomerular filtration rate 53 ± 19 ml/min/1.73 m2). Symptoms of depression were assessed by the Center for Epidemiologic Studies – Depression Scale (CES-D). After 1-night polysomnography each recording was visually scored and sleep macrostructure was analyzed.
Results:
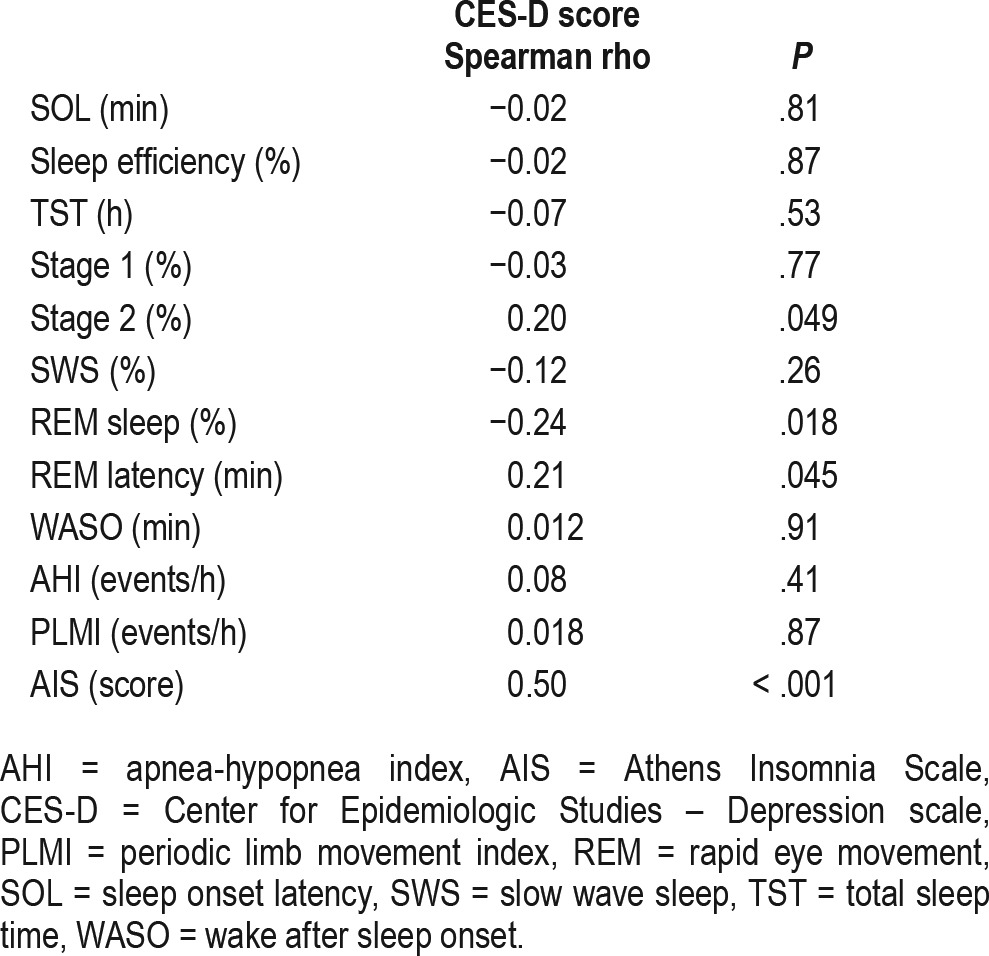
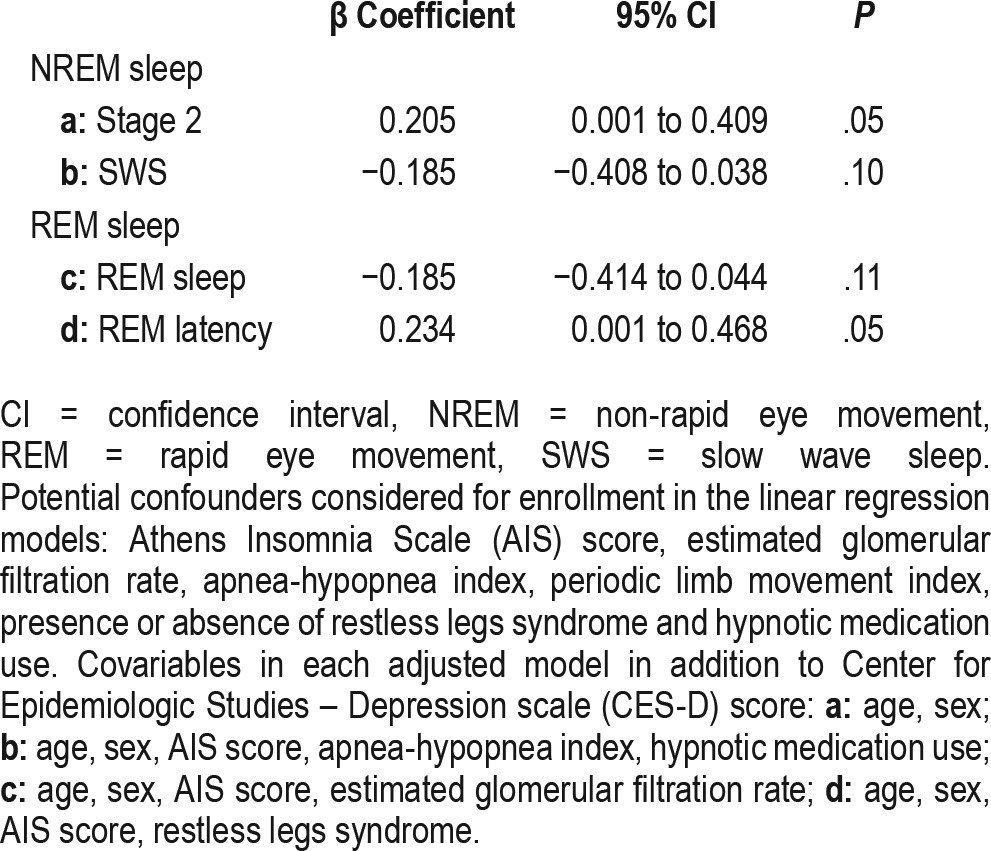
The CES-D score was significantly associated with the amount of stage 2 sleep (r = 0.20, P < .05), rapid eye movement (REM) latency (r = 0.21, P < .05) and REM percentage (r = −0.24, P < .05), but not with the amount of slow wave sleep (r = −0.12, P > .05). In multivariable linear regression models the CES-D score was independently associated with the amount of stage 2 sleep (β: 0.205; confidence interval: 0.001–0.409; P = .05) and REM latency (β: 0.234; confidence interval: 0.001–0.468; P = .05) after adjustment for potential confounders.
Conclusions:
Depressive symptoms among kTx recipients are associated with increased amount of stage 2 sleep and prolonged REM latency. Further studies are needed to confirm our findings and understand potential clinical implications.
Citation:
Ronai KZ, Szentkiralyi A, Lazar AS, Ujszaszi A, Turanyi C, Gombos F, Mucsi I, Bodizs R, Molnar MZ, Novak M. Depressive symptoms are associated with objectively measured sleep parameters in kidney transplant recipients. J Clin Sleep Med. 2017;13(4):557–564.
Keywords: depression, kidney transplant recipients, REM sleep, slow wave sleep, stage 2 sleep
INTRODUCTION
Depression is one of the most prevalent mental health conditions in patients with chronic kidney disease (CKD)1–4; furthermore, it is an important determinant of impaired quality of life.5,6 Among kidney transplant (kTx) recipients, depression is associated with reduced adherence and also with increased morbidity, graft loss, and mortality.7–13 Poor sleep and various sleep problems are also frequent complaints among kTx recipients.14,15 Earlier we reported that chronic insomnia was independently associated with the presence of depression in kTx recipients.16
In the general population there is a strong and bidirectional relationship between depression and sleep.17 This is also reflected in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition definition of major depression (MD),18 as sleep complaints are core symptoms of MD. Several objective sleep parameters are different in patients with MD than in healthy control patients. Patients with MD have decreased amounts of slow wave sleep (SWS) and shortened rapid eye movement (REM) latency with increased amounts of REM sleep.19–22
BRIEF SUMMARY
Current Knowledge/Study Rationale: Depression and sleep problems are very prevalent in kidney transplant recipients. However, the association between sleep structure and subjective depressive symptoms has not been investigated in this population.
Study Impact: We demonstrate, for the first time, a connection between depressive symptoms and objectively assessed sleep macrostructure among kidney transplant recipients. These results represent an important step in the understanding of the underlying processes linking depression and sleep among these patients.
The exact mechanisms and factors that may link depression to poor clinical outcomes among kTx recipients are not yet well defined. In a recent review focusing on kTx and depression,23 sleep is not mentioned. This may be due to the complete lack of polysomnography (PSG) studies that assess the association between sleep and depression in this population. The frequent co-occurrence of subjective sleep complaints and depression among kTx recipients16 suggests that the relationship between the two conditions may be similar to the association described in the general population.24–26 This highlights the clinical significance of sleep problems among kTx recipients, because they are potentially modifiable and interventions focusing on enhancing sleep may help to improve depressive symptoms.27
Despite the significance of depression among kTx recipients and its well-known associations with sleep problems in the general population, there is a lack of information regarding the connection of depression and sleep parameters assessed by PSG among kTx recipients. Evaluating sleep structure with PSG and its associations with depressive symptoms is an important step in the understanding of the pathophysiology behind the subjective symptoms. Thus, in this study, we aimed to investigate the macrostructure parameters of sleep and we hypothesized that, similar to patients with depression, less SWS, shortened REM sleep latency, and higher proportion of REM sleep are associated with depressive symptoms in this patient population.
METHODS
Sample of Patients and Data Collection
Data for this analysis were obtained from the SLeep disorders Evaluation in Patients after kidney Transplantation (SLEPT) study.28–34 Potentially eligible patients were selected from all prevalent adult kTx recipients (total clinic population; n = 1,214) who were regularly followed at a single outpatient academic transplant center, the kidney transplant clinic of the Department of Transplantation and Surgery at the Semmelweis University, Budapest, Hungary (Figure 1). All patients followed at the clinic on December 31, 2006 were considered for enrollment in the Malnutrition and INflammation In Transplant (MINIT-HU) study. After applying exclusion criteria (transplant received within less than 3 months, presence of active and acute respiratory disorder, acute infection, or hospitalization within 1 month, surgery within 3 months), 1,198 patients remained (base population). From this base population, we randomly selected and approached 150 patients (kTx study sample) using the simple random sampling strategy offered by SPSS 15.0 (IBM Corporation, Armonk, New York, United States). The creation of the cohort has been described previously.28–34 From these 150 eligible patients, 100 individuals agreed to participate and they underwent 1-night PSG (kTx participant sample). From this sample of 100 patients, 3 were excluded from our analysis because of a missing depression score and 2 were excluded because of antidepressant pharmacotherapy. Thus, the final kTx macrostructure sample included 95 patients whose PSG recordings were analyzed in this report. Demographic, anamnestic and questionnaire-based data were collected at enrollment, including age, sex, etiology of end-stage kidney disease (ESKD), transplantation-related data, medication use, and assessment of depression and insomnia symptoms.
Figure 1. Selection of patients.
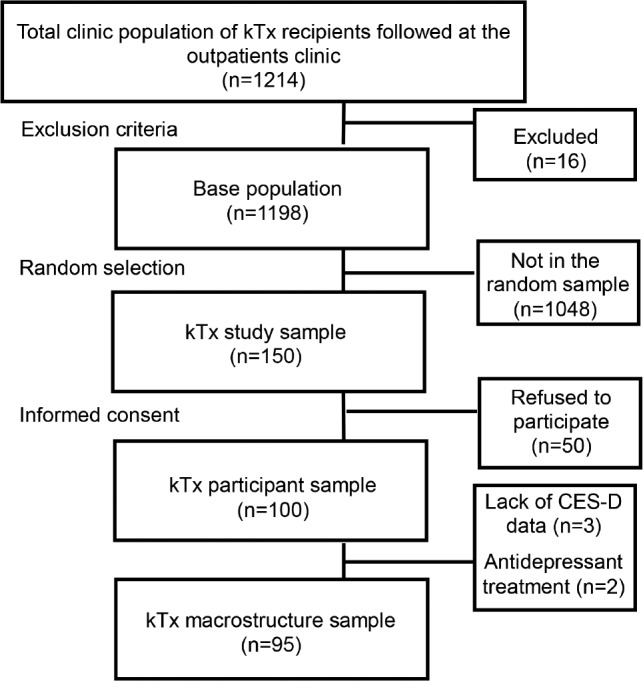
CES-D = Center for Epidemiologic Studies – Depression scale, kTx = kidney transplant.
Assessment of Depression
The Hungarian version of the Center for Epidemiologic Studies – Depression (CES-D) scale35 was prepared according to the recommended procedure36 and was validated by our team in Hungarian kTx recipients.37 The CES-D scale consists of 20 items and the score range is 0 to 60, with higher scores indicating lower mood. Subjects are asked to grade how frequently their complaints occurred and how long they lasted (rarely, 1 to 2 days, 3 to 4 days, 5 to 7 days) within the past week. In the current analysis the CES-D score was used to describe the severity of depressive symptoms in the sample. In addition, a cutoff score of 18 was used to estimate the frequency of clinically significant depression in patients with CKD as suggested by Hedayati et al.38
Polysomnography and Sleep Staging
Standard, attended overnight PSG was performed in acoustically isolated and video-monitored sleep laboratory equipped with four individual suits (SOMNOscreenTM PSG Tele, SOMNOmedics GmBH, Germany, CE0494). The following data were recorded: 5 electroencephalography channels (A1, A2, C3, C4, Cz), electrooculogram, chin electromyography, tibial electromyography, electrocardiography, airflow, thoracic– abdominal movements, pulse oximetry, tracheal sound (snoring), and body position. The ground and common reference electrodes were placed at Fpz and Cz, respectively. Electroencephalography signals were sampled and stored at 128 Hz, low- and high-pass filters were set at 35 Hz and 0.2 Hz, respectively. All recordings were performed on weekdays, the timing of “lights off” and “lights on” were uniformly set around 10:00 PM and 6:00 AM, respectively.
Recordings were manually scored by two somnologists (MZM, ASL). Sleep stages were determined in 30-second epochs according to Rechtschaffen and Kales.39 Sleep macro-structure was characterized by the following variables: sleep onset latency (time elapsed from “lights off ” to the first occurrence of sleep stage 2); total sleep time; wake after sleep onset (time spent awake from sleep onset to “lights on”), sleep efficiency (ratio of total sleep time over the time spent in bed); percentages of sleep stages 1, 2, SWS (sleep stages 3 and 4 combined) and percentage and latency of REM sleep. Respiratory events, periodic leg movements, and microarousals were scored according to standard criteria.40,41 Apnea was defined as the absence of airflow for more than 10 seconds; hypopnea was defined as a clearly discernible reduction in airflow for more than 10 seconds associated with an arousal and/or 3% reduction in oxygen saturation. The apnea-hypopnea index was defined as the number of apneas and hypopneas per hour of sleep. Periodic limb movements were defined as limb movements with duration of 0.5 to 5 seconds; intermovement interval of 5 to 90 seconds; and separation criteria for limb movements occurring in both legs: more than 5 seconds between onsets. The periodic limb movement index was defined as the number of limb movements per hours during sleep.
Assessment of Insomnia, Restless Legs Syndrome, and Comorbidities
The Athens Insomnia Scale (AIS) was used to assess sleep complaints and identify possible cases of insomnia.42,43 The AIS consists of 8 items, score range 0 to 24, with higher scores indicating worse sleep. Subjects are asked to grade the severity of the sleep complaints (absent, mild, severe, very severe) only if the particular complaint occurred at least 3 times per week during the last month. A cutoff score of 10 has been suggested for epidemiological studies, providing acceptable sensitivity and specificity to detect clinically significant insomnia.43 The English version of the AIS had been previously translated and validated by our group.44
Symptoms of restless legs syndrome (RLS) were identified by using the RLS Questionnaire (RLSQ) completed by the patients. The original version of the RLSQ had been carefully developed to cover the 4 diagnostic criteria of RLS and was shown to be a reliable screening instrument.45 The original instrument was used in an epidemiologic survey46 and the Hungarian version was used in our earlier studies involving different populations with CKD.47–49
Comorbidity was assessed by the modified Charlson Comorbidity Index50 completed by the main responsible transplant physician of the participant. Information about medication use was obtained from the questionnaires and the medical charts.
Laboratory Data
Laboratory data were extracted from the medical charts, including blood hemoglobin, serum albumin, and creatinine. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease EPIdemiology collaboration (CKD-EPI) formula.51
Transplantation and Donor-Related Data
Transplantation-related information collected included current medications, transplant and dialysis vintage (ie, time elapsed since transplantation or time spent on dialysis prior to transplantation), history of acute rejection, age and sex of donor, and history of delayed graft function. Time elapsed since the initiation of the first treatment for ESKD—cumulative ESKD time—was also calculated. Standard maintenance immunosuppressant therapy generally consisted of prednisolone, either cyclosporine A microemulsion formulation or tacrolimus, combined with mycophenolate mofetil or azathioprine, everolimus or sirolimus.
Ethics Approval
The study was approved by the Ethics Board of the Semmelweis University (April 2007). Before enrollment, patients received detailed verbal and written information about the aims and protocol of the study and provided signed informed consent.
Statistical Analysis
Statistical analysis was carried out using STATA 13.0 software. Continuous variables were compared using Student t test or the Mann-Whitney U test, as appropriate. Categorical variables were analyzed using the chi-square test or the Fisher exact test if the observation numbers were low. Bivariate analysis was performed using Pearson and Spearman rank correlation analysis.
We analyzed the association between the CES-D score and the selected sleep macrostructure parameters with multivariable linear regression analyses. The models were built with the sleep parameter as the dependent variable and the CES-D score as independent variable. In the models the potential confounders, which were selected based on theoretical considerations, were additionally subject to backward stepwise selection using a removal condition (P > .2): AIS score, graft function (eGFR), apnea-hypopnea index, periodic limb movement index, presence or absence of RLS and hypnotic medication use. Age, sex, and CES-D score were included in the models with forced selection. We used square root transformation to achieve normal distribution of the variable where it was necessary (REM latency). In all statistics, two-sided tests were used and P < .05 was considered statistically significant.
RESULTS
Demographic Data and Baseline Characteristics of the Sample
Of the 150 eligible patients (kTx study sample, see Methods section), 50 individuals (33%) refused to participate. Consequently, the kTx participant sample who underwent PSG included 100 kTx patients (Figure 1). There were no significant differences regarding age and sex between participants and those who refused to participate (data not shown). The basic characteristics (age, sex, eGFR, serum albumin) of the kTx participant sample were similar to the characteristics of the total clinic population (data not shown).
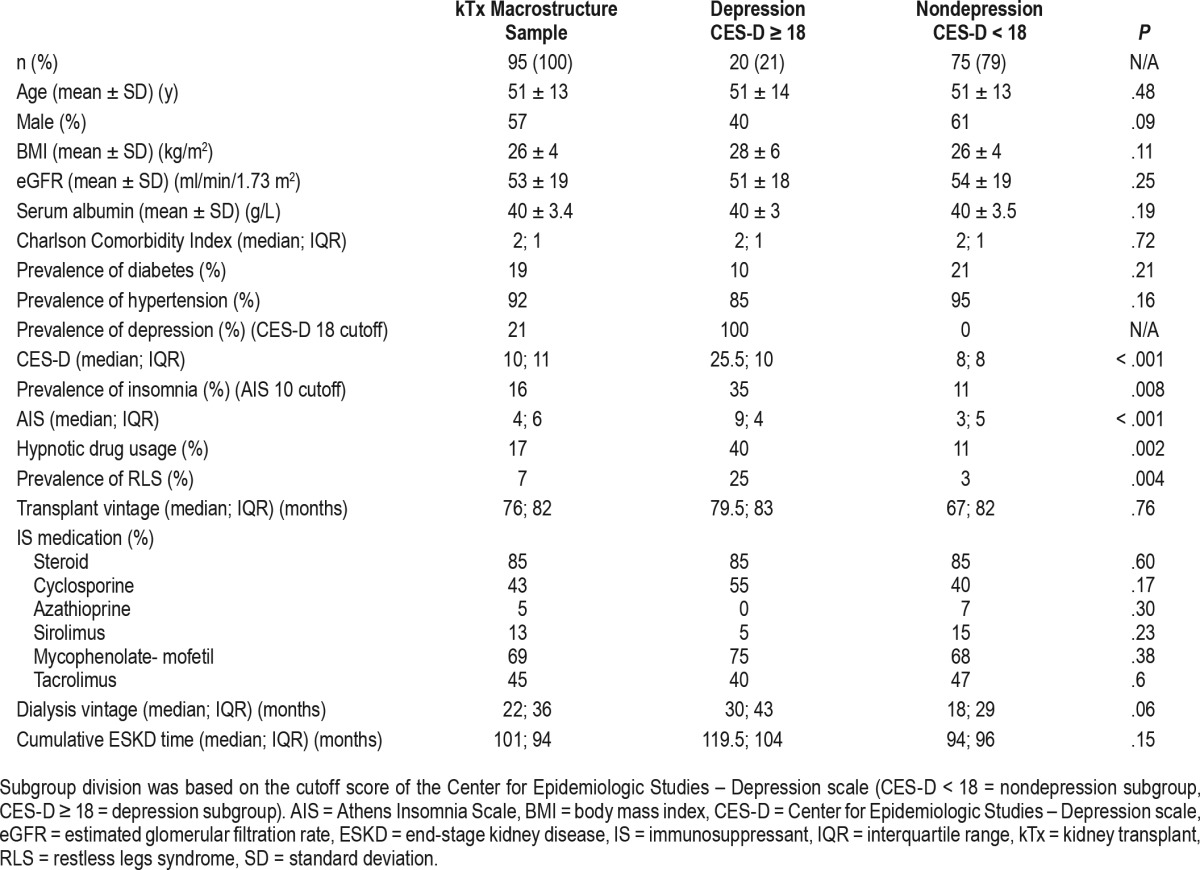
Of the 100 patients in the kTx participant sample we excluded 5 patients; 3 patients had missing CES-D scores and 2 patients were excluded because of treatment with antidepressant medication. Thus, the final kTx macrostructure sample included 95 patients (Figure 1). The demographic and laboratory parameters, the comorbid conditions, and transplantation related data of the kTx macrostructure sample are presented in Table 1.
Table 1.
Descriptive data of the kidney transplant macrostructure sample and of the subgroups depression and nondepression.
Prevalence and Severity of Depression and Associations with Demographics
The median of the CES-D score was 10 (interquartile range: 11) in the sample. One-fifth of the patients scored 18 or higher indicating high risk of depressive symptoms38 (Table 1). High depression risk was associated with high risk of insomnia and RLS; furthermore, these patients were taking significantly more hypnotic medication. Female sex and dialysis vintage were nearly significantly related to high depression risk.
Association between Depressive Symptoms and Sleep Quality
High risk for depression was significantly associated with less REM sleep and longer REM latency. Additionally, there was a tendency for more stage 2 sleep (Table 2). The proportion of SWS was not associated with high risk for depression (Table 2). CES-D score, a continuous measure of depression symptom severity, was significantly associated with subjective insomnia complaints as measured by the AIS score, with more stage 2 sleep, longer REM latency, and less REM sleep, but not with the proportion of SWS (Table 3).
Table 2.
Polysomnography data in the kidney transplant macrostructure sample (n = 95) and in the subgroups depression (n = 20) and nondepression (n = 75).
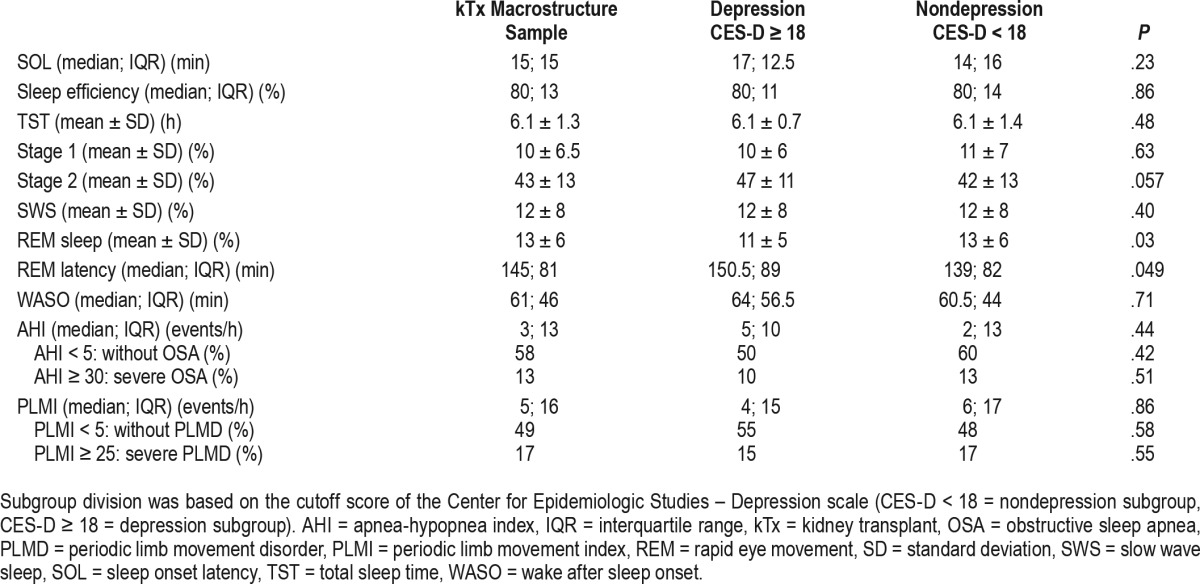
Table 3.
Correlations of the Center for Epidemiologic Studies – Depression scale score versus polysomnography parameters and Athens Insomnia Scale score.
Multivariable Analysis
To assess the independent association between depressive symptoms and sleep macrostructure we utilized multivariable analysis (Table 4). The selected sleep macrostructure parameters (proportion of SWS and REM sleep, REM sleep latency) were included in the multivariable models as dependent variables. Additionally, we also included the proportion of stage 2 sleep as dependent variable based on the results of the univariable analyses. Higher proportion of stage 2 sleep and longer REM latency were significantly associated with depression severity independent of other covariables. However, the association of REM sleep percentage with the CES-D score diminished after controlling for covariables. Similarly, the proportion of SWS was not associated with the CES-D score in the multivariable analysis.
Table 4.
Associations between the sleep parameters (dependent variable) and the Center for Epidemiologic Studies – Depression scale score (independent variable) in multivariable linear regression models.
DISCUSSION
As far as we know, this is the first study that assessed the association of depressive symptoms and sleep architecture of kTx recipients. Our main finding is that severity of depressive symptoms is associated with increased proportion of stage 2 sleep and longer REM latency, independently of important covariables. In our dataset, depressive symptoms were not associated with decreased SWS among kTx recipients.
There has been a growing interest in detailed assessment of sleep among patients with various stages of CKD52–54 and there is an increasing focus on patient-reported outcomes.55 However, these earlier studies were performed using in-home PSG and did not particularly focus on kTx recipients. Laboratory-assessed PSG studies among the kTx recipient population included much fewer patients (n1 = 18; n2 = 9; n3 = 34, respectively) and mainly focused on sleep apnea and the change of the sleep structure associated with apnea treatment or transplantation.56–58 Although some sleep macrostructure parameters were reported in these studies, little attention was paid to stage 2 sleep or REM sleep.
We found a significant association of stage 2 sleep with depressive symptoms among kTx recipients. Another study that assessed the sleep architecture of patients with CKD and ESKD reported significant association between increased stage 2 sleep and severe fatigue,52 without the significant involvement of SWS. This association, however, was not analyzed in multivariable analysis.
Interestingly, Smagula et al. also described increased stage 2 sleep (but not decreased SWS) associated with depressive symptoms among older men in a community-dwelling sample.59 They interpreted their result as a sign of accelerated age-related change in the sleep structure among this population. They also highlighted that the severity of depressive symptoms and the lack of treatment-seeking behavior of these participants were different from that of depressed inpatients. According to the authors' opinion,59 these differences might contribute to the sleep architecture variation60 they reported in this sample.
In fact, increased stage 2 sleep is not a characteristic feature of the sleep architecture of patients in whom MD was diagnosed.19–21 However, in a randomized placebo-controlled trial a significant improvement of low mood in olanzapine-treated participants was associated with changes mainly in sleep continuity measures and also the duration of stage 2 sleep, but not with the change of SWS.61
In this analysis we observed a surprisingly prolonged REM latency and decreased REM sleep among this population; however, we were not able to compare these results with healthy good sleepers Within this kTx population the prolongation of REM sleep latency and the consequent decrease of REM percentage may be both connected with depression.
In earlier PSG studies among kTx recipients the proportion of REM sleep was variable, from normal (21.6 ± 5.9%; 18.9 ± 8.3%)56,58 to low (14 ± 9.2%).57 REM latency was only reported in one study (133 ± 76 minutes).57 Interestingly, the proportion of REM sleep was also associated with declining renal function in patients with CKD, however, this association diminished after controlling for covariables.54 Depressive symptoms were not considered in any of the previously mentioned studies.
In a recent population-based study, longer REM latency was associated with depressive symptoms (also measured by the CES-D score) even after adjustment for age and sex.62 In the work of Smagula et al., shorter REM sleep was associated with more depressive symptoms.59 Although shortening of REM latency and more REM sleep are thought to be markers of depression, not every patient with depression is characterized by dysregulated REM sleep.19,21 Similarly, population-based studies sometimes report no association of REM parameters with depressive symptoms.63,64
Contrary to our expectation, we did not find a significant association between depressive symptoms and SWS among kTx recipients. Similarly, there was no association between SWS and depressive symptoms in a large community sample (n = 2,861).59 It is possible that the relationship between depressive symptoms and SWS is modified by unique characteristics of certain populations (such as age, comorbidities, medications, etc). The pathophysiology of the illness, metabolic changes, or the effect of medications may all be causes of variations in sleep architecture60,62 that may alter the expected patterns described in patients with MD.17,19,20 In this respect it is also interesting to note that SWS was higher in patients on dialysis compared to predialysis or transplanted patients.53,56 These results may indicate that the modality of the renal replacement therapy or the kidney disease itself may affect SWS and the regulation of non-rapid eye movement (NREM) sleep in some yet undefined way. It is also possible that the lack of significant association was due to the relatively small sample size and the consequently low statistical power.
One explanation of the observed sleep architecture variations may be that a sleep-protecting mechanism is overactivated during NREM sleep among kTx recipients. Such a mechanism would preserve SWS, but also would lead to the prolonged REM latency. Altered homeostasis of SWS could be investigated with exploring sleep microstructure including analyses of spindles, K-complexes, and cyclic alternating pattern.65,66
Several limitations of our study should be considered when interpreting the results. First, this is a cross-sectional study and this prevents us from drawing conclusions about causality or temporality. Second, we did not have a control group; therefore, we could not compare the sleep architecture with healthy sleepers. Third, it is possible that the impaired kidney function of kTx recipients might have influenced our findings. In this regard it is important to note that we adjusted our multivariable models for eGFR, a generally used parameter to characterize graft function. eGFR is reportedly associated with sleep structure in earlier stages of CKD.54 Fourth, we also considered sleep disorders as potential confounders while building our models. Importantly, these variables did not alter the associations we report.
In summary, we found that increased depressive symptom severity was significantly associated with higher stage 2 sleep percentage and prolonged REM latency in kTx recipients. These results may be related to the unique characteristics of this population and may suggest that a sleep protecting mechanism is associated with depressive symptoms among kTx recipients. Additionally, the altered NREM sleep regulation could partly be connected to the loss of REM sleep. Further research is needed to better understand the complex relationship between depressive symptoms and sleep structure among kTx recipients.
Based on our findings we propose that depression among this patient population should be treated as a different sub-type, and in case of pharmacological treatment the prolonged REM latency and low amount of REM sleep should be taken into consideration. Future studies are also warranted to define the most effective treatment for depression in this patient population. Furthermore, future research should also focus on whether psychotherapy targeting sleep problems would change these markers and improve depressive symptoms in kTx recipients.
DISCLOSURE STATEMENT
The analysis was performed at the Institute of Behavioural Sciences, Semmelweis University, Budapest, Hungary. This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are thankful for the help and support of the patients and the staff in the Department of Transplantation and Surgery and the Sleep Laboratory in the 1st Department of Internal Medicine, Semmelweis University, Budapest, Hungary.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AIS
Athens Insomnia Scale
- BMI
body-mass index
- CES-D scale
Center for Epidemiologic Studies – Depression scale
- CI
confidence interval
- CKD
chronic kidney disease
- CKD-EPI formula
Chronic Kidney Disease EPIdemiology formula
- eGFR
estimated glomerular filtration rate
- ESKD
end-stage kidney disease
- IQR
interquartile range
- IS
immunosuppressant
- kTx
kidney transplant
- MD
major depression
- MINIT-HU study
Malnutrition and INflammation In Transplant study
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PLMD
periodic limb movement disorder
- PLMI
periodic limb movement index
- PSG
polysomnography
- REM
rapid eye movement
- RLS
restless legs syndrome
- RLSQ
restless legs syndrome questionnaire
- SD
standard deviation
- SLEPT study
SLeep disorders Evaluation in Patients after kidney Transplantation study
- SOL
sleep onset latency
- SWS
slow wave sleep
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Cukor D, Peterson RA, Cohen SD, Kimmel PL. Depression in end-stage renal disease hemodialysis patients. Nat Clin Pract Nephrol. 2006;2(12):678–687. doi: 10.1038/ncpneph0359. [DOI] [PubMed] [Google Scholar]
- 2.Chilcot J, Wellsted D, Farrington K. Depression in end-stage renal disease: current advances and research. Semin Dial. 2010;23(1):74–82. doi: 10.1111/j.1525-139X.2009.00628.x. [DOI] [PubMed] [Google Scholar]
- 3.Bautovich A, Katz I, Smith M, Loo CK, Harvey SB. Depression and chronic kidney disease: a review for clinicians. Aust N Z J Psychiatry. 2014;48(6):530–541. doi: 10.1177/0004867414528589. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi: 10.1038/ki.2013.77. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs AZ, Molnar MZ, Szeifert L, et al. Sleep disorders, depressive symptoms and health-related quality of life--a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant. 2011;26(3):1058–1065. doi: 10.1093/ndt/gfq476. [DOI] [PubMed] [Google Scholar]
- 6.Lee YJ, Kim MS, Cho S, Kim SR. Association of depression and anxiety with reduced quality of life in patients with predialysis chronic kidney disease. Int J Clin Pract. 2013;67(4):363–368. doi: 10.1111/ijcp.12020. [DOI] [PubMed] [Google Scholar]
- 7.Novak M, Molnar MZ, Szeifert L, et al. Depressive symptoms and mortality in patients after kidney transplantation: a prospective prevalent cohort study. Psychosom Med. 2010;72(6):527–534. doi: 10.1097/PSY.0b013e3181dbbb7d. [DOI] [PubMed] [Google Scholar]
- 8.Zelle DM, Dorland HF, Rosmalen JG, et al. Impact of depression on long-term outcome after renal transplantation: a prospective cohort study. Transplantation. 2012;94(10):1033–1040. doi: 10.1097/TP.0b013e31826bc3c8. [DOI] [PubMed] [Google Scholar]
- 9.Rocha G, Poli de Figueiredo CE, d'Avila D, Saitovitch D. Depressive symptoms and kidney transplant outcome. Transplant Proc. 2001;33(7–8):3424. doi: 10.1016/s0041-1345(01)02476-9. [DOI] [PubMed] [Google Scholar]
- 10.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, Maclean JR, Kasiske BL. Depressive disorder in renal transplantation: an analysis of Medicare claims. Am J Kidney Dis. 2008;51(5):819–828. doi: 10.1053/j.ajkd.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Szeifert L, Molnar MZ, Ambrus C, et al. Symptoms of depression in kidney transplant recipients: a cross-sectional study. Am J Kidney Dis. 2010;55(1):132–140. doi: 10.1053/j.ajkd.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Noohi S, Khaghani-Zadeh M, Javadipour M, et al. Anxiety and depression are correlated with higher morbidity after kidney transplantation. Transplant Proc. 2007;39(4):1074–1078. doi: 10.1016/j.transproceed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Jindal RM, Neff RT, Abbott KC, et al. Association between depression and nonadherence in recipients of kidney transplants: analysis of the United States renal data system. Transplant Proc. 2009;41(9):3662–3666. doi: 10.1016/j.transproceed.2009.06.187. [DOI] [PubMed] [Google Scholar]
- 14.Sabbatini M, Crispo A, Pisani A, et al. Sleep quality in renal transplant patients: a never investigated problem. Nephrol Dial Transplant. 2005;20(1):194–198. doi: 10.1093/ndt/gfh604. [DOI] [PubMed] [Google Scholar]
- 15.Burkhalter H, Brunner DP, Wirz-Justice A, et al. Self-reported sleep disturbances in renal transplant recipients. BMC Nephrol. 2013;14:220. doi: 10.1186/1471-2369-14-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novak M, Molnar MZ, Ambrus C, et al. Chronic insomnia in kidney transplant recipients. Am J Kidney Dis. 2006;47(4):655–665. doi: 10.1053/j.ajkd.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MJ, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015;10(1):17–23. doi: 10.1016/j.jsmc.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 19.Riemann D, Berger M, Voderholzer U. Sleep and depression--results from psychobiological studies: an overview. Biol Psychol. 2001;57(1):67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 20.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007;(433):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 21.Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013;17(5):377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Arfken CL, Joseph A, Sandhu GR, Roehrs T, Douglass AB, Boutros NN. The status of sleep abnormalities as a diagnostic test for major depressive disorder. J Affect Disord. 2014;156:36–45. doi: 10.1016/j.jad.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Chilcot J, Spencer BW, Maple H, Mamode N. Depression and kidney transplantation. Transplantation. 2014;97(7):717–721. doi: 10.1097/01.TP.0000438212.72960.ae. [DOI] [PubMed] [Google Scholar]
- 24.Hartz AJ, Daly JM, Kohatsu ND, Stromquist AM, Jogerst GJ, Kukoyi OA. Risk factors for insomnia in a rural population. Ann Epidemiol. 2007;17(2):940–947. doi: 10.1016/j.annepidem.2007.07.097. [DOI] [PubMed] [Google Scholar]
- 25.van Mill JG, Hoogendijk WJ, Vogelzangs N, van Dyck R, Penninx BW. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry. 2010;71(3):239–246. doi: 10.4088/JCP.09m05218gry. [DOI] [PubMed] [Google Scholar]
- 26.Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Med. 2007;8(Suppl 4):S15–20. doi: 10.1016/S1389-9457(08)70004-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen HY, Cheng IC, Pan YJ, et al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int. 2011;80(4):415–422. doi: 10.1038/ki.2011.151. [DOI] [PubMed] [Google Scholar]
- 28.Molnar MZ, Lazar AS, Lindner A, et al. Sleep apnea is associated with cardiovascular risk factors among kidney transplant patients. Clin J Am Soc Nephrol. 2010;5(1):125–132. doi: 10.2215/CJN.04030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovesdy CP, Czira ME, Rudas A, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant. 2010;10(2):2644–2651. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Molnar MZ, Czira ME, et al. Associations between serum leptin level and bone turnover in kidney transplant recipients. Clin J Am Soc Nephrol. 2010;5(12):2297–2304. doi: 10.2215/CJN.03520410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovesdy CP, Mucsi I, Czira ME, et al. Association of serum phosphorus level with anemia in kidney transplant recipients. Transplantation. 2011;91(8):875–882. doi: 10.1097/TP.0b013e3182111edf. [DOI] [PubMed] [Google Scholar]
- 32.Molnar MZ, Czira ME, Rudas A, et al. Association between the malnutrition-inflammation score and post-transplant anaemia. Nephrol Dial Transplant. 2011;26(6):2000–2006. doi: 10.1093/ndt/gfq690. [DOI] [PubMed] [Google Scholar]
- 33.Molnar MZ, Czira ME, Rudas A, et al. Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;58(1):101–108. doi: 10.1053/j.ajkd.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 34.Molnar MZ, Keszei A, Czira ME, et al. Evaluation of the malnutrition-inflammation score in kidney transplant recipients. Am J Kidney Dis. 2010;56(1):102–111. doi: 10.1053/j.ajkd.2010.02.350. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 36.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25(24):3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 37.Zoller R, Molnar MZ, Mucsi I, et al. Factorial invariance and validity of the Hungarian version of the Center for Epidemiological Studies-Depression (CES-D) Scale [abstr] Qual Life Res. 2005;14(9):2036. [Google Scholar]
- 38.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69(9):1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 39.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 40.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 41.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 42.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 43.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the Athens Insomnia Scale. J Psychosom Res. 2003;55(3):263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 44.Novak M, Mucsi I, Shapiro CM, Rethelyi J, Kopp MS. Increased utilization of health services by insomniacs--an epidemiological perspective. J Psychosom Res. 2004;56(5):527–536. doi: 10.1016/j.jpsychores.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Allen RP EC. Validation of a diagnostic questionnaire for the restless legs syndrome (RLS) {abstract} Neurology. 2001;56(Suppl 3):4A. [Google Scholar]
- 46.Nichols DA, Allen RP, Grauke JH, et al. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med. 2003;163(19):2323–2329. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 47.Mucsi I, Molnar MZ, Rethelyi J, et al. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol Dial Transplant. 2004;19(7):1815–1822. doi: 10.1093/ndt/gfh130. [DOI] [PubMed] [Google Scholar]
- 48.Mucsi I, Molnar MZ, Ambrus C, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20(3):571–577. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- 49.Szentkiralyi A, Molnar MZ, Czira ME, et al. Association between restless legs syndrome and depression in patients with chronic kidney disease. J Psychosom Res. 2009;67(2):173–180. doi: 10.1016/j.jpsychores.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis. 2005;46(1):136–142. doi: 10.1053/j.ajkd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jhamb M, Liang K, Yabes J, et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol. 2013;38(6):489–495. doi: 10.1159/000356939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roumelioti ME, Argyropoulos C, Pankratz VS, et al. Objective and subjective sleep disorders in automated peritoneal dialysis. Can J Kidney Health Dis. 2016;3:6. doi: 10.1186/s40697-016-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogna A, Forni Ogna V, Haba Rubio J, et al. Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus Cohort. Sleep. 2016;39(4):945–953. doi: 10.5665/sleep.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jhamb M, Tamura MK, Gassman J, et al. Design and rationale of health-related quality of life and patient-reported outcomes assessment in the Frequent Hemodialysis Network trials. Blood Purif. 2011;31(1-3):151–158. doi: 10.1159/000321855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beecroft JM, Zaltzman J, Prasad R, Meliton G, Hanly PJ. Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease. Nephrol Dial Transplant. 2007;22(10):3028–3033. doi: 10.1093/ndt/gfm309. [DOI] [PubMed] [Google Scholar]
- 57.Jurado-Gamez B, Martin-Malo A, Rodriguez-Benot A, Munoz-Cabrera L, Cosano Povedano A, Aljama P. Kidney transplantation improves sleep-related breathing in hemodialysis patients. Blood Purif. 2008;26(6):485–490. doi: 10.1159/000157373. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigues CJ, Marson O, Togeiro SM, Tufik S, Ribeiro AB, Tavares A. Sleep-disordered breathing changes after kidney transplantation: a polysomnographic study. Nephrol Dial Transplant. 2010;25(6):2011–2015. doi: 10.1093/ndt/gfp752. [DOI] [PubMed] [Google Scholar]
- 59.Smagula SF, Reynolds CF, Ancoli-Israel S, et al. Sleep architecture and mental health among community-dwelling older men. J Gerontol B Psychol Sci Soc Sci. 2015;70(5):673–681. doi: 10.1093/geronb/gbt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kudlow PA, Cha DS, Lam RW, McIntyre RS. Sleep architecture variation: a mediator of metabolic disturbance in individuals with major depressive disorder. Sleep Med. 2013;14(10):943–949. doi: 10.1016/j.sleep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Lazowski LK, Townsend B, Hawken ER, Jokic R, du Toit R, Milev R. Sleep architecture and cognitive changes in olanzapine-treated patients with depression: a double blind randomized placebo controlled trial. BMC Psychiatry. 2014;14:202. doi: 10.1186/1471-244X-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luik AI, Zuurbier LA, Whitmore H, Hofman A, Tiemeier H. REM sleep and depressive symptoms in a population-based study of middle-aged and elderly persons. J Sleep Res. 2015;24(3):305–308. doi: 10.1111/jsr.12273. [DOI] [PubMed] [Google Scholar]
- 63.Castro LS, Castro J, Hoexter MQ, et al. Depressive symptoms and sleep: a population-based polysomnographic study. Psychiatry Res. 2013;210(3):906–912. doi: 10.1016/j.psychres.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 64.Kravitz HM, Avery E, Sowers M, et al. Relationships between menopausal and mood symptoms and EEG sleep measures in a multi-ethnic sample of middle-aged women: the SWAN sleep study. Sleep. 2011;34(9):1221–1232. doi: 10.5665/SLEEP.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 66.Halasz P, Bodizs R, Parrino L, Terzano M. Two features of sleep slow waves: homeostatic and reactive aspects--from long term to instant sleep homeostasis. Sleep Med. 2014;15(10):1184–1195. doi: 10.1016/j.sleep.2014.06.006. [DOI] [PubMed] [Google Scholar]


