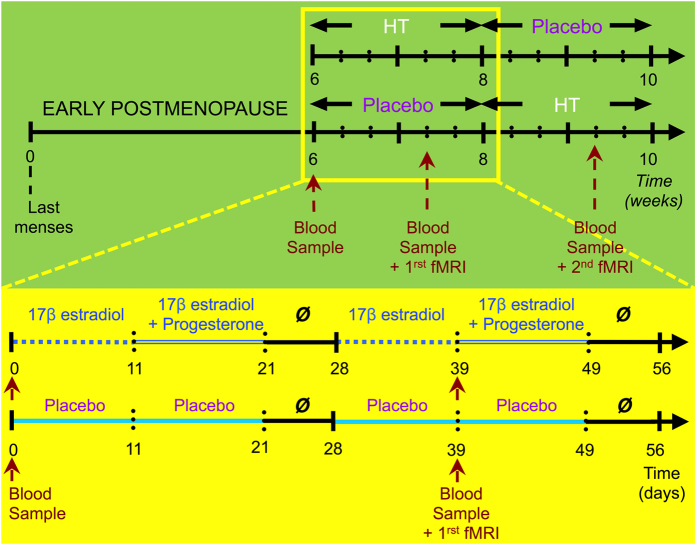
Figure 1. Experimental design.
Early postmenopausal women were enrolled in a double blind, randomized, placebo-controlled crossover study. They took a daily pill of either HT (respectively placebo) for two cycles of 28 days each, followed by two consecutive months of placebo (respectively HT). For the first 11 days of a ‘restored’ menstrual cycle (under HT), pills contained 17β estradiol (2 mg/day). From the 12th to the 21th day, the pills contained an addition of 100 mg/day of progesterone. This was followed by a week washout period. This cycle was repeated for a second month (see yellow rectangle). After these two months, a new cycle started: women receiving HT first were administrated with a placebo containing 2 mg/day of inactive substance for the first 11 days and 102 mg/day for the following 10 days. This cycle was repeated during a last cycle of 28 days. The order of receiving HT and placebo was randomly assigned and counter-balanced. Blood samples were collected at the beginning of the study and on each day of scanning (once under HT and once under Placebo).