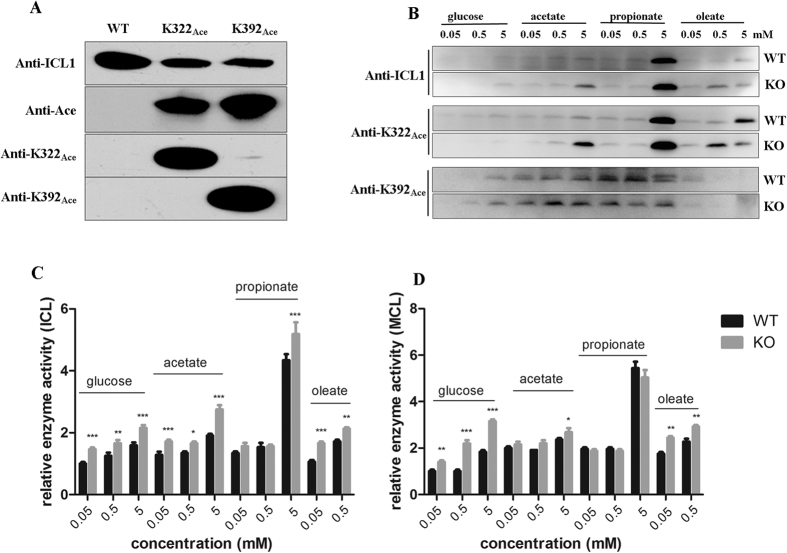
Figure 4. Expression and acetylation of ICL1 in M. tb under different carbon sources.
(A) Western blot analysis of purified WT or ICL1 proteins in which K322 or K392 was acetylated. Equal amount of purified proteins (1 μg per lane) was analyzed by Western blot. Antibodies raised against ICL1 (anti-ICL1), K322 or K392 acetylated peptides (anti-K322Ace, anti-K392Ace), or acetyllysine (anti-Ace) were used. (B) Western blot analysis of cell extracts of WT and ΔnpdA (KO) grown in different carbon sources at various concentrations. The cultures were grown in 7H9 media supplemented with 0.5% BSA, 0.085% NaCl and different carbon source at indicated concentration at 37 °C for 8 days. Cell extracts (20 μg per lane) was analyzed by Western blot with appropriate antibodies. Duplicate SDS-PAGE gel stained with Coomassie blue was used as the loading control (Supplementary Fig. S4A and B). Results are representative of three independent experiments. (C) Isocitrate lyase (ICL) activities of M. tb cultures grown in different carbon sources at various concentrations. The cell extracts of WT and ΔnpdA (KO) grown in different carbon sources at indicated concentrations were analyzed. The data were normalized against the ICL activity of WT cultures grown in 0.05 mM glucose. (D) Methylcitrate lyase (MCL) activities of M. tb cultures grown in different carbon sources at various concentrations. The cell extracts of WT and ΔnpdA (KO) grown in different carbon sources at indicated concentrations were analyzed. The data were normalized against the MCL activity of WT cultures grown in 0.05 mM glucose. For both (C) and (D), the data are plotted as mean ± s.d. (n = 3). Two-way ANOVA was performed to compare WT and ΔnpdA at each condition. *p < 0.05; **p < 0.01; ***p < 0.001.