Fig. 2.
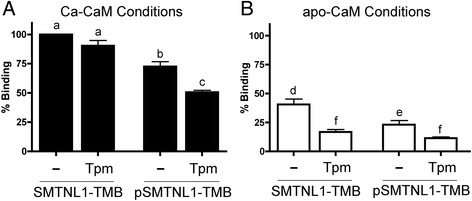
Interdependency of SMTNL1 phosphorylation, calcium and tropomyosin on calmodulin-binding. SMTNL1-TMB (200 μg, 7 nmol) was incubated with an equimolar amount of Tpm. The mixture was then incubated with CaM-Sepharose (40 μL; ligand density of ~10–14 μmol/mL) in the presence (a; Ca-CaM, 5 mM CaCl2) or absence (b; apo-CaM, 1 mM EDTA) of calcium. Some experiments were completed with SMTNL1-TMB that had been previously phosphorylated with PKA. After washing, the retention of SMTNL1-TMB or phosphorylated SMTNL1-TMB was analyzed. The band densities were quantified and binding to CaM-Sepharose expressed as percentage of SMTNL1-TMB recovered under maximal binding conditions (i.e., Ca-CaM in the absence of Tpm). All experiments are n = 3–5 and were analyzed by one-way ANOVA with Tukey’s post hoc analysis. Different letters indicate significant differences among groups (a,b,c: comparison among all Ca-CaM conditions; d,e,f: comparison among all apo-CaM conditions; p < 0.05)
