Fig. 1.
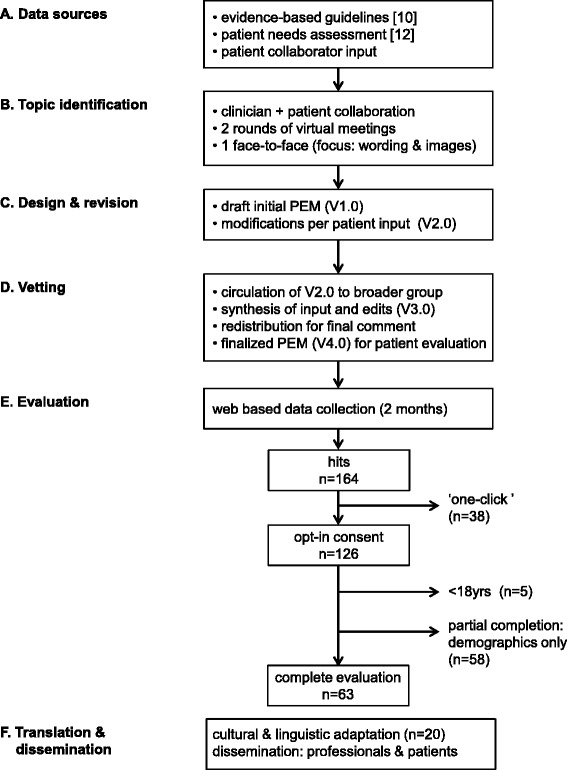
Study Schema. PEM were co-created in a multi-step process. (a) Three main sources were used for PEM development. (b) Members of the Patient Advocacy Working Group and patient collaborators identified topics for the PEM in an iterative process. (c) The initial draft was created and revised based on patient input. (d) PEM (V2.0) was circulated to the Clinical Working Group and Genetics Working Group members for comment and revised accordingly with patient validation in two rounds. (e) PEM (V4.0) were evaluated by patients recruited via social media (private/closed Facebook groups), patient support meetings and via RareConnect [12]. (f) Following evaluation materials were culturally adapted and translated to 20 languages and distributed in avenues targeting healthcare professionals and patients. PEM: patient education materials, V: version
