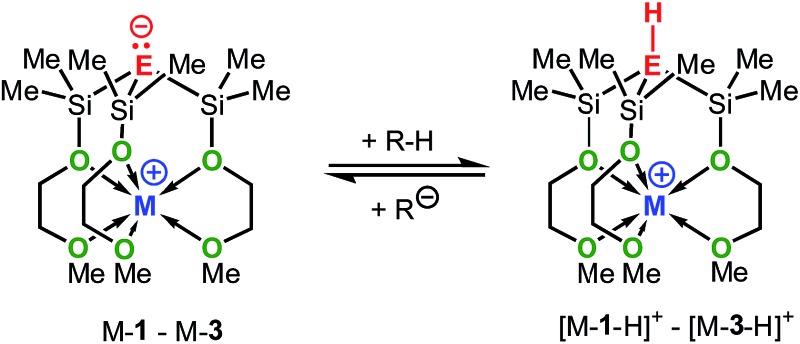
Table 1. Estimated pK a values of M-1–M-3 (M = Li, Na, K) measured in C6D6 and THF-D8 .
| ||
| Base | pK a (C6D6) a | pK a (THF-D8) a |
| Na-1/(2.2.2-cryptand) | — | 29.2 |
| K-1 | 22.2 | 24.5 |
| Na-1 | 22.5 | 23.6 |
| Li-1 | 23.0 | 23.6 |
| K-2/(2.2.2-cryptand) | ≈26 (±1) b | — |
| K-2 | 18.7 | 22.3 |
| Na-2 | ≈16.0 c | 18.8 |
| Li-2 | 18.0 | 19.8 |
| K-3/(2.2.2-cryptand) | 22.9 | — |
| K-3 | <16 d | 18.0 |
| Na-3 | <16 d | <16 d |
| Li-3 | <16 d | ≈16.0 c |
a The calculated pK a values refer to the conjugated acids.
b The pK a value was estimated to be 26 ± 1, as no deprotonation of Ph3PCH2PPh3 [pK a(DMSO) = 29.9] but 100% deprotonation of 9-Bu t -fluorene [pK a(DMSO) = 24.4] was observed.
c Approximately 10% deprotonation of 9-Ph-fluorene.
d No conversion with 9-Ph-fluorene.
