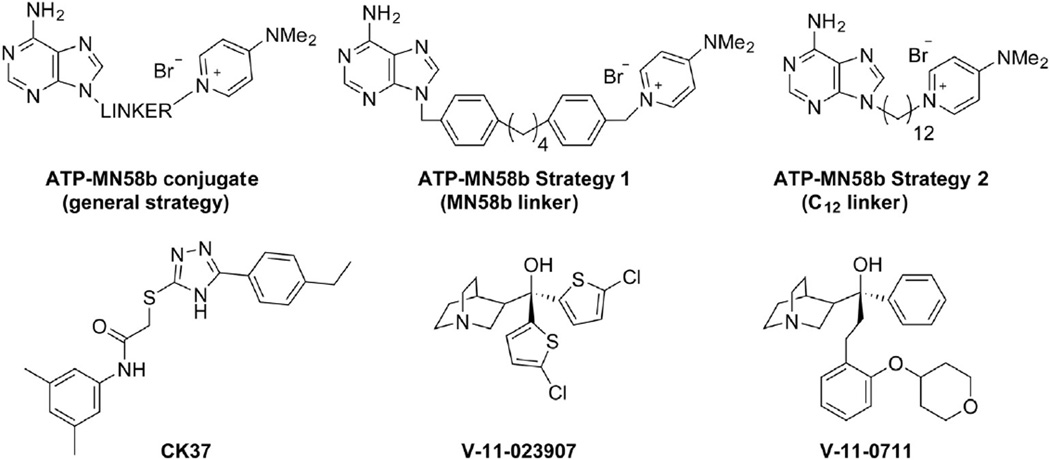
Fig. 6.
Asymmetric ChoKα inhibitors. A rational design approach to improve potency involved the addition of adenine moieties to MN58b (ATP-MN58b conjugate) in an effort to bind the choline and ATP binding sites simultaneously. Sahún-Roncero et al. [126] used an MN58b linker group in a mixed ATP-MN58b inhibitor (Strategy 1), while Trousil et al. [124] independently found a 12-carbon linkage (ATP-MN58b Strategy 2) was capable of inhibition in vitro. Next generation ChoKα inhibitors were developed using computational (CK37) and compound library (V-11-023,907 and V-11-0711) screening approaches.