Abstract
Diabetes is prevalent among solid organ transplant recipients and is universal among islet transplant recipients. Whereas diabetes is often considered to result in an immune-compromised state, the impact of chronic hyperglycemia on host alloimmunity is not clear. Potential immune-modifying effects of obesity, autoimmunity, or diabetogenic agents like streptozotocin may confound understanding alloimmunity in experimental models of diabetes. Therefore, we sought to determine the role of chronic hyperglycemia due to insulinopenia on alloimmunity using the nonautoimmune, spontaneously diabetic H-2b–expressing C57BL/6 Ins2Akita mice (Akita). Akita mice harbor a mutated Ins2 allele that dominantly suppresses insulin secretion, resulting in lifelong diabetes. We used BALB/c donors (H-2d) to assess alloimmunization and islet transplantation outcomes in Akita recipients. Surprisingly, chronic hyperglycemia had little effect on primary T-cell reactivity after alloimmunization. Moreover, Akita mice readily rejected islet allografts, and chronic hyperglycemia had no impact on the magnitude or quality of intragraft T-cell responses. In contrast, allospecific IgM and IgG were significantly decreased in Akita mice after alloimmunization. Thus, whereas diabetes influences host immune defense, hyperglycemia itself does not cause generalized alloimmune impairment. Our data suggest that immune compromise in diabetes due to hyperglycemia may not apply to cellular rejection of transplants.
Introduction
Diabetes is more prevalent among solid organ transplant recipients (based on public Organ Procurement and Transplantation Network [OPTN] data) than in the general population (1) and is universal among islet and pancreas transplant recipients. Although diabetes impairs patient immunity to pathogens (2), it is not known whether alloimmunity is altered in chronic hyperglycemia. The heterogeneity of human diabetes and the use of potent immune-suppressive drugs to prevent cellular alloresponses preclude addressing this question using clinical transplant data.
Numerous animal studies in both chemically induced (3–8) and obese (9–11) diabetic models report that diabetes decreases cellular (3–7,9–11) and humoral immunity (3,5,8), including alloimmunity (4,7,9–11). However, confounding immune effects of streptozotocin (12,13) and disrupted leptin signaling (14) complicate interpretation of these studies. Similarly, since autoimmunity can influence alloimmunity (15), the NOD model is also not suitable to address how chronic hyperglycemia itself affects alloimmunity. Therefore, we turned to the spontaneously diabetic, nonautoimmune C57BL/6 Ins2Akita (Akita) model (16) to study alloimmunity. A single mutant Ins2 allele in Akita mice dominantly suppresses insulin secretion due to a protein-misfolding defect (17). Although Akita mice are reported to reject islet allografts (18,19), a careful analysis of alloresponses is lacking in this newer animal model of diabetes. Using the Akita model, we evaluated the intrinsic impact of chronic hyperglycemia resulting from insulinopenia on primary cellular and humoral alloresponses.
Research Design and Methods
Animals
C57BL/6 Ins2Akita (Akita; H-2b), C57BL/6J, and BALB/cByJ (H-2d) mice were purchased from The Jackson Laboratory. Male Akita mice treated with an islet isograft were crossed with C57BL/6 female mice and male offspring heterozygous for the Akita polymorphism (Ins2Akita/+) were used as diabetic recipients (blood glucose ≥20 mmol/L) at 8–12 weeks of age. Nondiabetic littermates (B6) were used as controls. Drs. Sang-Mo Kang (University of California San Francisco) and Anita Chong (The University of Chicago) provided C57BL/6 T-cell receptor transgenic 4C (20) and TCR75 (21) mice, respectively. Mice were bred and housed in AAALAC-accredited facilities. The University of Colorado Institutional Care and Use Committee approved all practices and procedures.
Assessment of Polyclonal T-Cell Responses to Local Alloimmunization
Mice received 106 BALB/cByJ splenocytes injected subcutaneously into each hind footpad. After 5 days, the draining popliteal lymph nodes (PLNs) were harvested for flow cytometry.
Staining and Flow Cytometry
Cells were stained with fixable live/dead discriminating dyes (BD or eBioscience) and antibodies to surface markers and intracellular proteins (using the FoxP3 staining kit; eBioscience). CD4 (RM4-5), CD8 (53-6.72), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD90.2 (30-H12), Vb13 (MR12-3), Vb8.3 (1B3.3), FoxP3 (FJK-16s), IFNγ (XMG1.2), and Ki67 (B56) antibodies were obtained from BD or eBioscience. For intracellular cytokine staining, T cells were restimulated in Eagle’s minimum essential medium containing phorbol myristic acid (40 ng/mL) and ionomycin (500 ng/mL) in the presence of brefeldin A (1 μg/mL) for 4 h. Samples were acquired on a BD LSR-II flow cytometer and analyzed using FlowJo software.
Assessment of Adoptively Transferred Allospecific T-Cell Receptor Transgenic Cells
CD45.1 congenic 4C and TCR75 mice were depleted of CD8+ T cells using anti-CD8 (clone 2.43). 4C (106) and TCR75 (104) cells were transferred into CD45.2+ Akita mice and nondiabetic littermates. Alloimmunization and harvest were performed as above. To identify transferred cells, live singlet CD4+ cells were gated on CD45.1 and then Vb13+ (4C) by Vb8.3+ (TCR75).
Streptozotocin Treatment
Where indicated, nondiabetic mice were given a single retro-orbital injection of streptozotocin (180 mg/kg; Sigma-Aldrich or Cayman). After 3 days, mice with two blood glucose readings ≥20 mmol/L were considered diabetic. To control for streptozotocin, a group of diabetic Akita mice was also treated.
Islet Isolation and Transplantation
Islets from female BALB/cByJ retired breeders were isolated using Cizyme (Vitacyte) and Lympholyte 1.1 (Cedarlane). Islet equivalents (400–450) were grafted beneath the left kidney capsule. Blood glucose was measured to confirm engraftment (two consecutive readings ≤10 mmol/L) and rejection (two consecutive readings ≥20 mmol/L).
Analysis of Graft-Infiltrating Cells
On day 7 posttransplant, a group of grafts was harvested, digested using dispase II (Roche), and further dissociated between frosted glass slides. Graft-infiltrating cell numbers were estimated using counting beads (eBioscience). Restimulation and staining were performed as above.
Assessment of Antidonor Antibody Response
Mice received 3 × 107 live BALB/cByJ splenocytes injected intraperitoneally or an islet allograft. Serum was harvested on days 0, 7, 14, and 21 or on the day of rejection of islet allografts (day 9–18). B6 mice were matched to a rejecting Akita. H-2d–expressing A20 target cells were exposed to sera (diluted 1:5) from immunized mice for 20 min at 37°C. Serum from a hyperimmune mouse was used as a positive control. Cells were then stained with secondary dye–conjugated anti-mouse IgM (Il/41) and anti-IgG antibody (11-4011-85; eBioscience).
Statistics
Data were graphed and statistically analyzed using Prism software. Statistical tests included multiple Student t tests with Bonferroni-Dunn corrections (alloimmunizations), Mann-Whitney U test (allograft survival), and two-way ANOVA tests with Bonferroni correction (graft-infiltrate analysis).
Results
No Effect of Chronic Diabetes on Polyclonal T-Cell Responses to Local Allogeneic Stimulation In Vivo
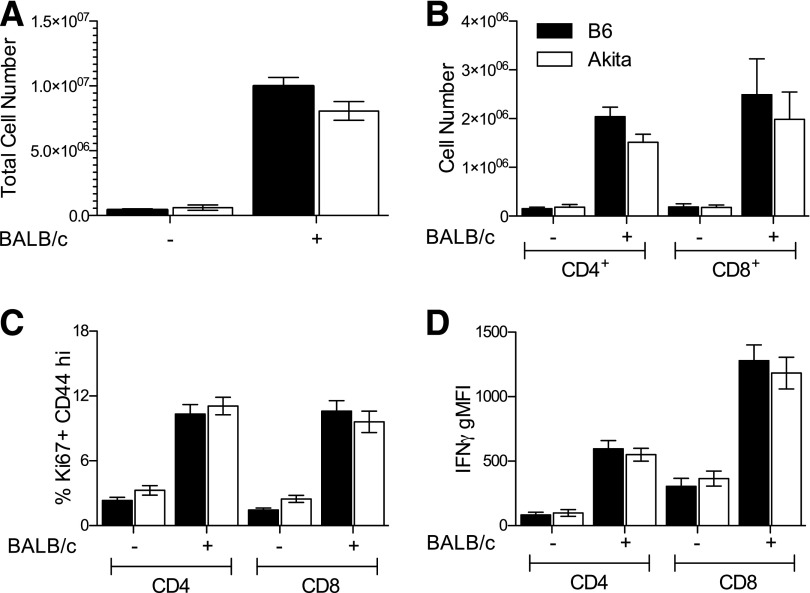
Naïve Akita mice demonstrate splenic lymphopenia but have otherwise normal B- and T-cell distribution and normal T-cell memory phenotypes (Supplementary Fig. 1). To determine whether chronic hyperglycemia affected cellular alloreactivity, we immunized Akita mice and nondiabetic littermate controls with allogeneic splenocytes and analyzed the T-cell response in the draining PLN. Surprisingly, chronic diabetes did not significantly affect total cell numbers (Fig. 1A) or CD4+ and CD8+ T-cell numbers (Fig. 1B) despite a tendency toward fewer cells.
Figure 1.
No effect of chronic hyperglycemia on primary T-cell responses to alloantigen. A: PLN cell counts in alloimmunized mice. B: CD4+ and CD8+ cell counts in PLN. C: Frequency of responding Ki67+CD44hi cells in PLN. D: Geometric mean fluorescence intensity (gMFI) of IFNγ in Ki67+CD44hi cells. Black bars, B6; white bars, Akita. n = 6–8 per group from seven independent experiments. No significant differences after multiple Student t tests with Bonferroni-Dunn corrections.
We next determined whether diabetes affected markers of T-cell antigen experience (CD44), proliferation (Ki67), or effector cytokine production (IFNγ). Again, chronic diabetes did not affect the frequency of responding Ki67+CD44hi cells (Fig. 1C) or their production of IFNγ (Fig. 1D).
No Effect of Chronic Diabetes on Adoptively Transferred Direct or Indirect Alloreactive T-Cell Responses
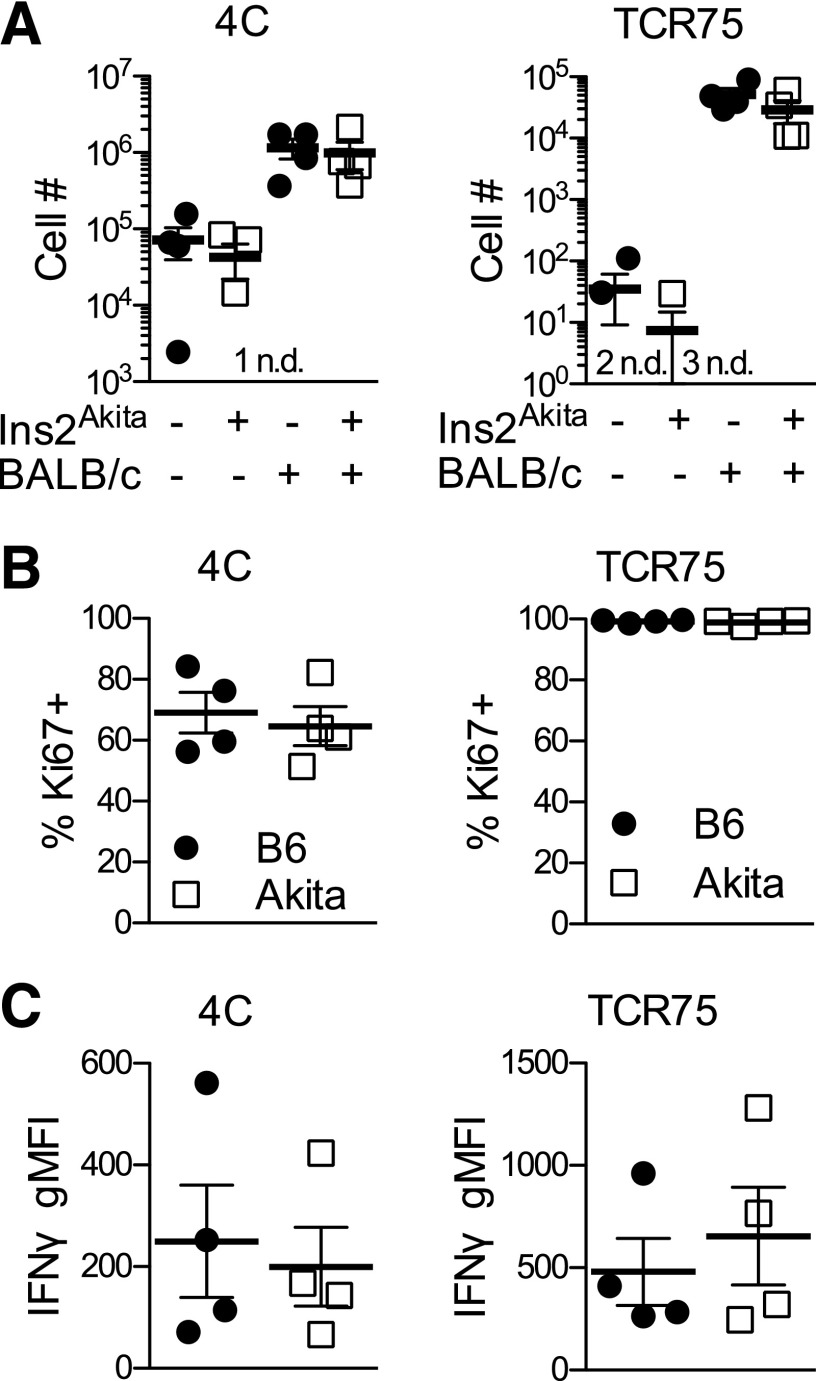
Although polyclonal alloresponses appeared normal, we next determined whether T cells recognizing alloantigens directly via donor antigen-presenting cells (only recently exposed to hyperglycemia) would be differentially affected by hyperglycemia versus T cells recognizing alloantigen processed and presented indirectly on host antigen-presenting cells (chronically exposed to hyperglycemia). We used 4C T-cell receptor (TCR) transgenic mice (20) to model directly alloreactive T cells and TCR75 TCR transgenic mice (21) to model indirectly alloreactive T cells. Hyperglycemia did not affect the numbers (Fig. 2A), proliferation (Fig. 2B), or IFNγ production (Fig. 2C) of adoptively transferred direct (4C) or indirect (TCR75) alloreactive TCR transgenic cells in draining PLNs of immunized mice. Thus, regardless of the pathway of allorecognition, T-cell responses were unaffected by chronic diabetes.
Figure 2.
No effect of chronic hyperglycemia on responses of either direct or indirect alloreactive TCR transgenic adoptively transferred cells. A: Number of direct (4C) and indirect (TCR75) alloreactive T cells in draining PLNs of Akita mice and euglycemic littermates 5 days after local alloimmunization. B: Frequency of proliferating (Ki67+) direct (4C) and indirect (TCR75) cells. C: Geometric mean fluorescence intensity (gMFI) of IFNγ in direct (4C) and indirect (TCR75) cells. Black circles, B6; white squares, Akita. Number of samples with no detectable cells in unchallenged mice indicated adjacent to “n.d.” n = 4 per group from four independent experiments. No significant differences between immunized groups after multiple Student t tests with Bonferroni-Dunn corrections.
No Effect of Chronic Diabetes on Islet Allograft Rejection
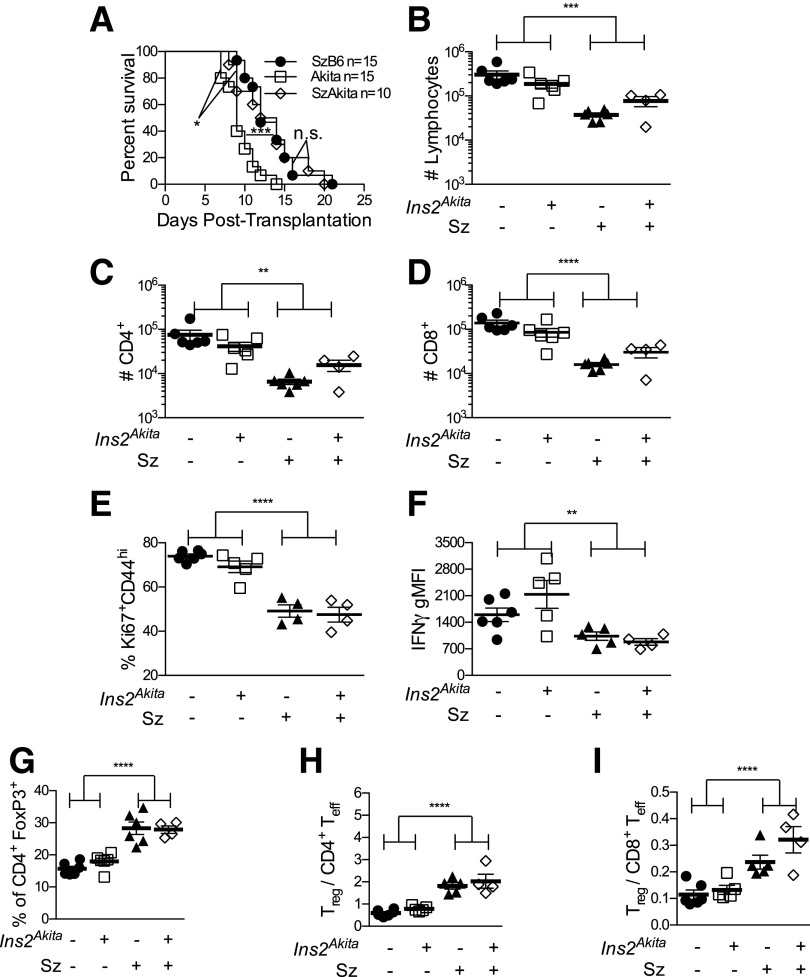
We next determined whether chronic diabetes affected acute islet allograft rejection. Since elevated blood glucose signifies graft rejection, Akita littermates were treated with streptozotocin to induce diabetes (SzB6). To control for streptozotocin (12,13,22,23), some Akita mice were also treated with this agent (SzAkita). Akita recipients rejected allografts more rapidly than SzB6 recipients (Fig. 3A). Since SzAkita mice rejected with the same tempo as SzB6 recipients, chronic hyperglycemia alone could not account for apparent accelerated rejection in Akita recipients.
Figure 3.
No impact of chronic hyperglycemia on cellular responses to islet allografts. A: Survival curves of islet allograft acute rejection. n = 10–15. ***P < 0.001, Akita vs. SzB6; *P < 0.05, Akita vs. SzAkita; SzB6 vs. SzAkita not significant by nonparametric Mann-Whitney U test. B: Total numbers of graft-infiltrating cells at day 7. Numbers of CD4+ (C) and CD8+ T cells (D) within CD90+ graft-infiltrating cells. E: Frequency of responding Ki67+CD44hi cells within CD90+CD8+ graft-infiltrating cells. F: Geometric mean fluorescence intensity (gMFI) of IFNγ in CD90+CD8+FoxP3−Ki67+CD44hi T cells. G: Frequency of FoxP3+ cells within CD90+CD4+ graft-infiltrating cells. Ratio of CD90+CD4+FoxP3+ regulatory T cells to CD90+FoxP3−Ki67+CD44hi CD4+ (H) and CD8+ (I) effector T cells. Black circles, B6; white squares, Akita; black triangles, SzB6; white diamonds, SzAkita. In B–I, n = 4–6 per group from five independent experiments. Two-way ANOVA with Bonferroni corrections: **P < 0.01; ***P < 0.001; ****P < 0.0001.
Streptozotocin, but Not Chronic Hyperglycemia, Alters the Magnitude of the Cellular Response to Islet Allografts
To further distinguish the effects of chronic hyperglycemia from those of streptozotocin, we examined intragraft T cells in B6, Akita, SzB6, and SzAkita recipients at day 7 posttransplant. Chronic hyperglycemia did not affect the total number of intragraft live cells or CD4+ and CD8+ cells, although all mice exposed to streptozotocin had fewer graft-infiltrating cells (Fig. 3B–D). Thus, chronic hyperglycemia did not affect the magnitude of the cellular alloresponse.
Streptozotocin, but Not Chronic Hyperglycemia, Alters the Phenotype of T Cells Responding to Islet Allografts
We next determined whether chronic hyperglycemia affected intragraft T-cell activation or regulation. Although prior chronic hyperglycemia did not affect the frequency of Ki67+CD44hi responding T cells within islet allografts, streptozotocin decreased the frequency of CD8+Ki67+CD44hi cells (Fig. 3E) by one-third. Prior chronic hyperglycemia did not affect IFNγ expression, but streptozotocin reduced the level of IFNγ production in Ki67+CD44hi T cells (Fig. 3F) by nearly 50% (49%). Whereas chronic hyperglycemia did not affect the frequency of FoxP3+ regulatory T cells, streptozotocin doubled FoxP3+ regulatory T-cell frequency (Fig. 3G). As a result, streptozotocin (but not chronic hyperglycemia) increased the ratio of regulatory T cells (CD4+FoxP3+) to effector T cells (FoxP3−Ki67+CD44hi) in both CD4+ (Fig. 3H) and CD8+ (Fig. 3I) compartments. Thus, streptozotocin treatment, but not prior chronic hyperglycemia, shifted the quality of the intragraft T-cell response.
Impaired Antibody Response to Alloantigen In Vivo
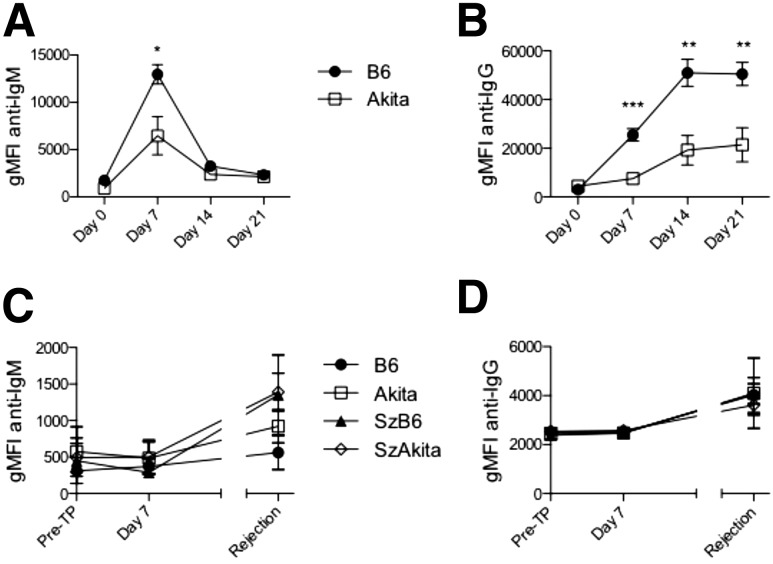
Finding no major differences in cellular alloresponses in chronic diabetes, we next assessed humoral alloresponses. Akita mice immunized with allogeneic splenocytes showed decreased allospecific IgM (Fig. 4A) and IgG (Fig. 4B) at 7, 14, and 21 days postchallenge. Only low levels of allospecific antibody (Fig. 4C and D) were generated in response to islet allografts themselves in both diabetic and euglycemic animals, which is consistent with B-cell dispensability for acute islet allograft rejection (24). Nevertheless, the marked reduction in alloantibody production after alloimmunization in Akita mice indicates that chronic hyperglycemia results in decreased humoral alloimmunity.
Figure 4.
Impaired humoral response to alloantigen in chronic diabetes. H-2d–expressing A20 target cells were exposed to serum from alloimmunized mice (A and B, n = 9–16 per group from four independent experiments) or islet allograft recipients (C and D, n = 4–8 per group from two independent experiments). A: Geometric mean fluorescence intensity (gMFI) of anti-IgM. B: gMFI of anti-IgG. C: gMFI of anti-IgM. D: gMFI of anti-IgG. Multiple Student t tests with Bonferroni-Dunn corrections: *P < 0.05; **P < 0.01; ***P < 0.001. Pre-TP, pretransplant.
Discussion
We have shown that chronic diabetes, without autoimmunity or chemical induction, specifically impairs humoral, but not cellular, alloimmunity in vivo. Using four different experimental approaches (polyclonal alloimmunization, TCR transgenic alloimmunization, islet allograft survival, and islet allograft infiltrate analysis), we have demonstrated that primary cellular alloresponses are not affected by chronic hyperglycemia. Our finding of splenic lymphopenia in chronic hyperglycemia (Supplementary Fig. 1) was consistent with other studies (7) but did not preclude normal cellular alloresponses. In contrast, chronic hyperglycemia resulted in decreased allospecific IgM and IgG responses. Our data do not determine whether the hyporeactive humoral immunity is T- or B-cell intrinsic, although others have reported normal T-independent but impaired T-dependent antibody responses in the streptozotocin model (3). Our finding of selective impairment of humoral versus cellular immunity in chronic hyperglycemia differs from previously published results in streptozotocin (3,5) and obese models of diabetes (6,10,11), which indicated impairment of both cellular and humoral immunity in diabetic animals. Diabetes-independent immune modulating effects of streptozotocin and obesity could potentially explain why cellular immunity is decreased in those models but remains normal in Akita mice. Although our interest was to study chronic hyperglycemia as an independent variable influencing alloimmunity, we should note that insulinopenia in Akita mice akin to type 1 diabetes could also potentially affect results. As such, concomitant insulinopenia could potentially limit the general relevance of this study to all patients with diabetes, many of whom are insulin resistant and/or hyperinsulinemic. This latter point raises the additional prospect that the form of diabetes in the general transplant patient population could potentially influence host alloimmunity. Whereas the current study suggests that alloimmunity is not impaired in the hyperglycemic, insulin-sensitive recipient (like many patients with type 1 diabetes), it is less clear whether this response is impacted by the insulin-resistant, hyperinsulinemic environment of the patient with type 2 diabetes. As such, this concept would encourage close interactions between diabetologists and transplant physicians. Nevertheless, our results qualify the tenet of immune compromise in diabetes by identifying a specific immune response (the primary cellular alloresponse) that is not compromised in chronic hyperglycemia.
The nature of the underlying alloresponse of transplant recipients (including recipients with diabetes) may become more relevant as the transplant immunology field seeks to replace lifelong, broad immunosuppression with short-term peri-transplant tolerance-promoting therapies. Although we find here that chronic diabetes does little to prevent primary cellular alloimmunity, we have previously reported decreased anti-CD154–induced tolerance to islet allografts in Akita mice (18). The normal capacity for acute islet allograft rejection combined with decreased capacity for tolerance induction in chronic hyperglycemia unfortunately constitutes a formidable challenge for the future of tolerance induction in patients with diabetes. This hitherto unappreciated challenge exists independent of autoimmunity, which further compounds the difficulty of tolerance induction in patients with type 1 diabetes.
Finally, our results highlight the importance of model selection in experimental islet transplantation studies. Despite early indications (13) and subsequent confirmation (12,22,23) of the immune modulating properties of streptozotocin, this drug has become the standard model for tolerance induction studies in islet transplantation (25). Our results confirm and extend those of previous groups by showing that streptozotocin itself dramatically alters the magnitude and quality of the cellular alloresponse within actual islet allografts. Due to these marked effects, our results reinforce the caveats related to the use of streptozotocin in transplant studies and support the use of the Akita model as a potential alternative nonautoimmune model of diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Philip Pratt and the Barbara Davis Center Flow Cytometry and Islet Isolation Core Facilities. The authors also thank Drs. Sang-Mo Kang (University of California, San Francisco) and Anita Chong (The University of Chicago) for generously providing TCR transgenic mice. The authors thank Drs. Rachel Friedman and Eric Clambey (University of Colorado Anschutz Medical Campus) for careful review of the manuscript.
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants RO1 DK099187 and 1 UC4 DK104223 (R.G.G.) and by National Institute of Allergy and Infectious Diseases training grant 5T32AI007405 (N.H.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.H.B. designed and performed experiments, analyzed data, and assisted with the manuscript. M.K.N. assisted in data analysis and interpretation and manuscript review. K.S.B. assisted in experiments and discussed results. R.G.G. designed experiments, interpreted data, and assisted with manuscript preparation. M.C. assisted in experimental design and data analysis. R.G.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0218/-/DC1.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA, Department of Health and Human Services, 2014 [Google Scholar]
- 2.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003;26:510–513 [DOI] [PubMed] [Google Scholar]
- 3.Saiki O, Negoro S, Tsuyuguchi I, Yamamura Y. Depressed immunological defence mechanisms in mice with experimentally induced diabetes. Infect Immun 1980;28:127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavelić K, Slijepcević M, Pavelić J. Recovery of immune system in diabetic mice after treatment with insulin. Horm Metab Res 1978;10:381–386 [DOI] [PubMed] [Google Scholar]
- 5.Handwerger BS, Fernandes G, Riehm T, Sutherland DE, Brown DM. Alterations in immunological function in streptozotocin-induced murine diabetes mellitus: correction by islet cell transplantation. Clin Immunol Immunopathol 1984;32:275–284 [DOI] [PubMed] [Google Scholar]
- 6.Mahmoud AA, Rodman HM, Mandel MA, Warren KS. Induced and spontaneous diabetes mellitus and suppression of cell-mediated immunologic responses. Granuloma formation, delayed dermal reactivity and allograft rejection. J Clin Invest 1976;57:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo B, Chan WF, Lord SJ, et al. . Diabetes induces rapid suppression of adaptive immunity followed by homeostatic T-cell proliferation. Scand J Immunol 2007;65:22–31 [DOI] [PubMed] [Google Scholar]
- 8.Ishibashi T, Kitahara Y, Harada Y, Harada S, Takamoto M, Ishibashi T. Immunologic features of mice with streptozotocin-induced diabetes: depression of their immune responses to sheep red blood cells. Diabetes 1980;29:516–523 [DOI] [PubMed] [Google Scholar]
- 9.Moraes-Vieira PM, Bassi EJ, Larocca RA, et al. . Leptin deficiency modulates allograft survival by favoring a Th2 and a regulatory immune profile [published correction appears in Am J Transplant 2013;13:2231]. Am J Transplant 2013;13:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes G, Handwerger BS, Yunis EJ, Brown DM. Immune response in the mutant diabetic C57BL/Ks-dt+ mouse. Discrepancies between in vitro and in vivo immunological assays. J Clin Invest 1978;61:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandel MA, Mahmoud AA. Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J Immunol 1978;120:1375–1377 [PubMed] [Google Scholar]
- 12.Muller YD, Golshayan D, Ehirchiou D, et al. . Immunosuppressive effects of streptozotocin-induced diabetes result in absolute lymphopenia and a relative increase of T regulatory cells. Diabetes 2011;60:2331–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols WK, Spellman JB, Vann LL, Daynes RA. Immune responses of diabetic animals. Direct immunosuppressant effects of streptozotocin in mice. Diabetologia 1979;16:51–57 [DOI] [PubMed] [Google Scholar]
- 14.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 2014;192:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalache S, Lakhani P, Heeger PS. Effects of preexisting autoimmunity on heart graft prolongation after donor-specific transfusion and anti-CD154. Transplantation 2014;97:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46:887–894 [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Takeuchi T, Tanaka S, et al. . A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999;103:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop NH, Beard KS, Gill RG. Resistance of spontaneously diabetic Ins2(akita) mice to allograft tolerance induced by anti-CD154 therapy. Transplant Proc 2014;46:2007–2009 [DOI] [PubMed] [Google Scholar]
- 19.Mathews CE, Langley SH, Leiter EH. New mouse model to study islet transplantation in insulin-dependent diabetes mellitus. Transplantation 2002;73:1333–1336 [DOI] [PubMed] [Google Scholar]
- 20.Brennan TV, Hoang V, Garrod KR, et al. . A new T-cell receptor transgenic model of the CD4+ direct pathway: level of priming determines acute versus chronic rejection. Transplantation 2008;85:247–255 [DOI] [PubMed] [Google Scholar]
- 21.Honjo K, Xu Xy, Bucy RP. CD4+ T-cell receptor transgenic T cells alone can reject vascularized heart transplants through the indirect pathway of alloantigen recognition. Transplantation 2004;77:452–455 [DOI] [PubMed] [Google Scholar]
- 22.Takayama Y, Ichikawa T, Maki T. Effect of STZ administration on islet isograft and allograft survival in NOD mice. Diabetes 1993;42:324–329 [DOI] [PubMed] [Google Scholar]
- 23.Koulmanda M, Qipo A, Auchincloss H Jr, Smith RN. Effects of streptozotocin on autoimmune diabetes in NOD mice. Clin Exp Immunol 2003;134:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KM, Yeh H, Zhao G, et al. . B-cell depletion improves islet allograft survival with anti-CD45RB. Cell Transplant 2014;23:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantarelli E, Citro A, Marzorati S, Melzi R, Scavini M, Piemonti L. Murine animal models for preclinical islet transplantation: No model fits all (research purposes). Islets 2013;5:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.