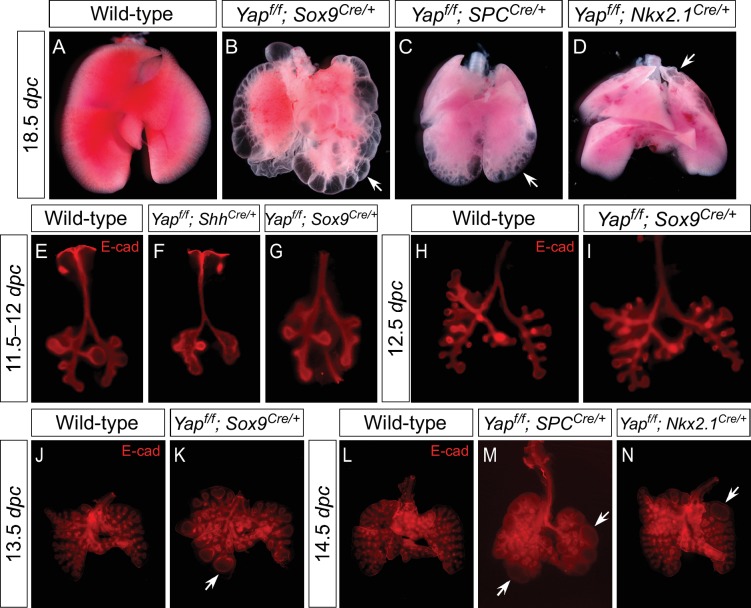
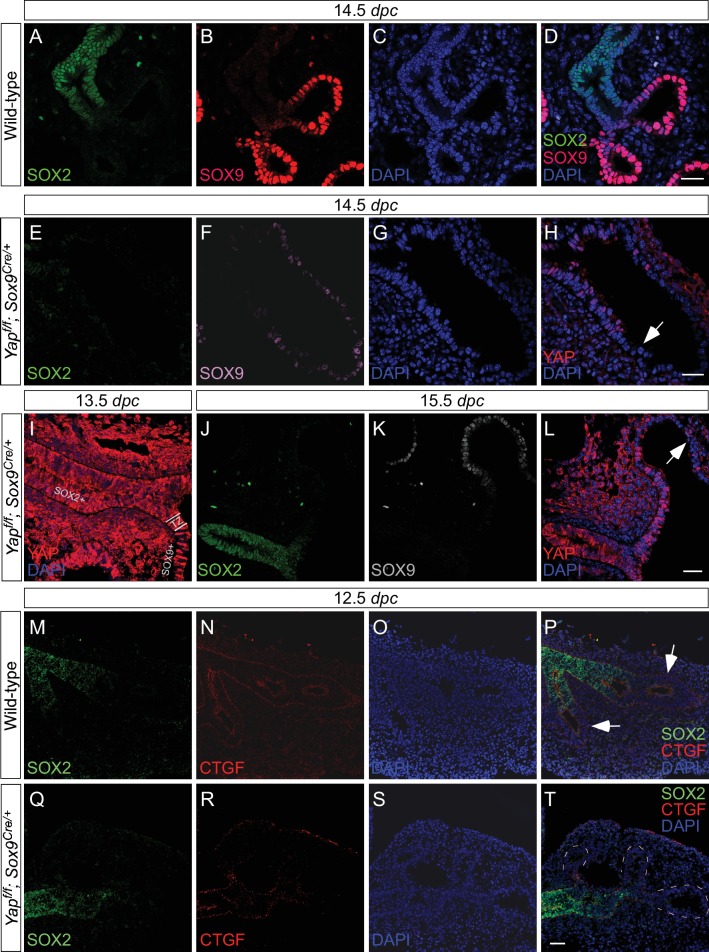
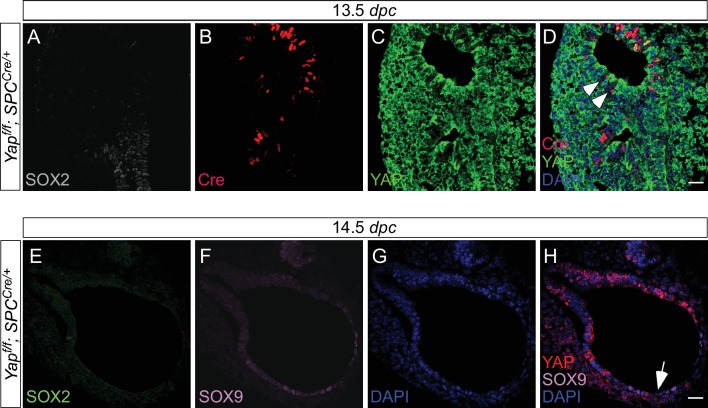
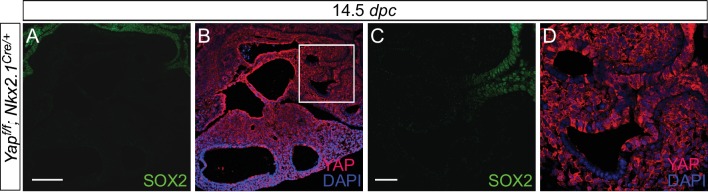
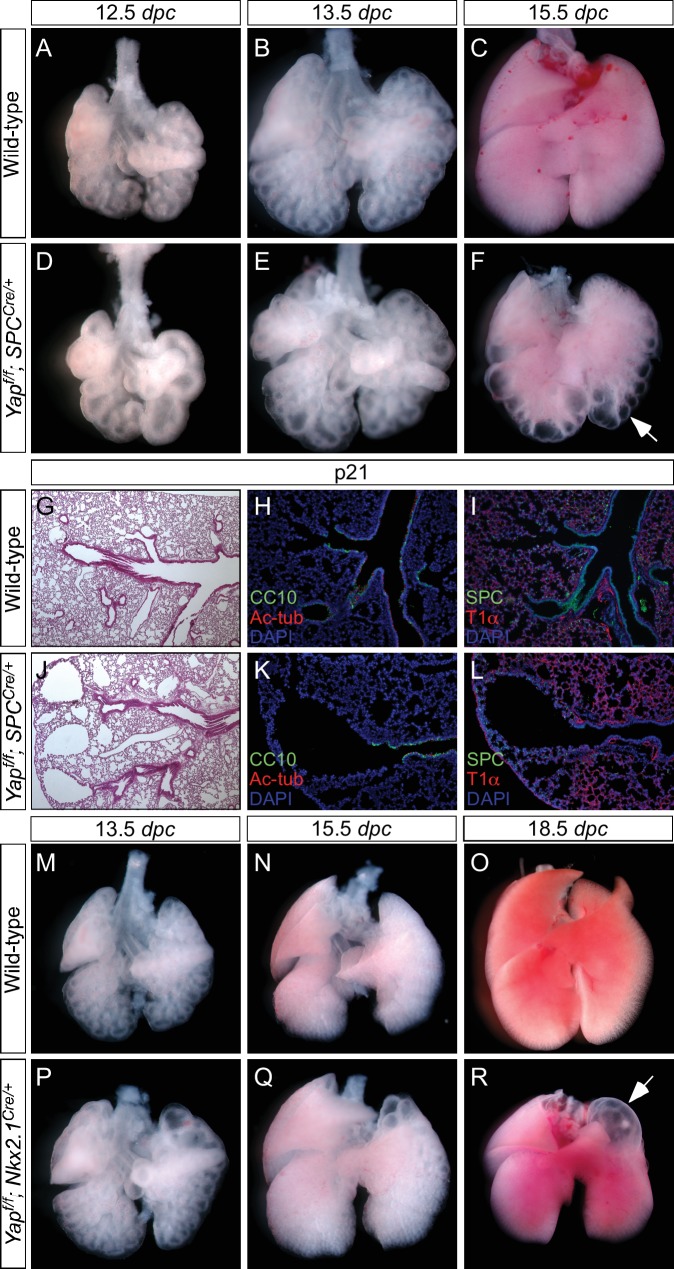
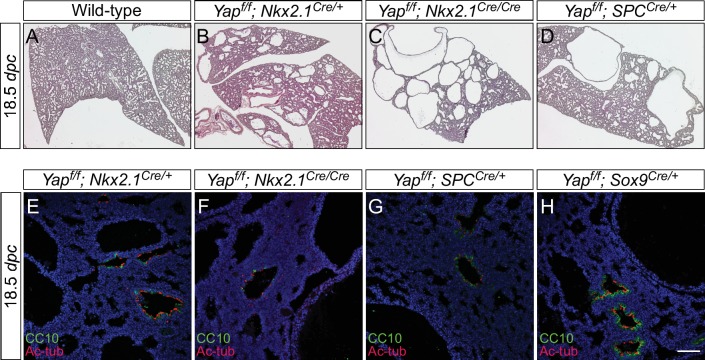
Figure 3. Regional loss of epithelial Yap leads to localized lung cysts.
(A–D) Ventral view of dissected lungs from wild-type, Yapf/f; Sox9Cre/+, Yapf/f; spcCre/+ and Yapf/f; Nkx2.1Cre/+ mice at 18.5 dpc. Lung cysts in Yapf/f; Sox9Cre/+ and Yapf/f; spcCre/+ mice were largely confined to the distal airway (arrows in B,C), while lung cysts were found in the upper lobes (arrow in D) of Yapf/f; Nkx2.1Cre/+ mice. The location of lung cyst formation is correlated with the sites of strong Cre activity and Yap removal. This suggests that loss of Yap at a given region leads to localized lung cysts. (E–N) Whole-mount immunostaining of dissected lungs from wild-type, Yapf/f; ShhCre/+, Yapf/f; Sox9Cre/+, Yapf/f; spcCre/+ and Yapf/f; Nkx2.1Cre/+ mice at the stages indicated. Lung epithelium was visualized by E-cadherin (E-cad). While defective branching was apparent in Yapf/f; ShhCre/+ lungs at 11.5 dpc, branching defects and cyst formation in Yapf/f; Sox9Cre/+ lungs did not appear until 13.5 dpc. Cyst formation in Yapf/f; Sox9Cre/+ lungs was confined to the distal airways (arrow in K). Similarly, cyst formation in Yapf/f; spcCre/+ lungs was detected primarily in the distal airways (arrows in M). By contrast, cyst formation was found in the upper lobes (arrow in N) of Yapf/f; Nkx2.1Cre/+ lungs. This suggests that loss of Yap at a given region leads to localized lung cysts. All views are ventral.