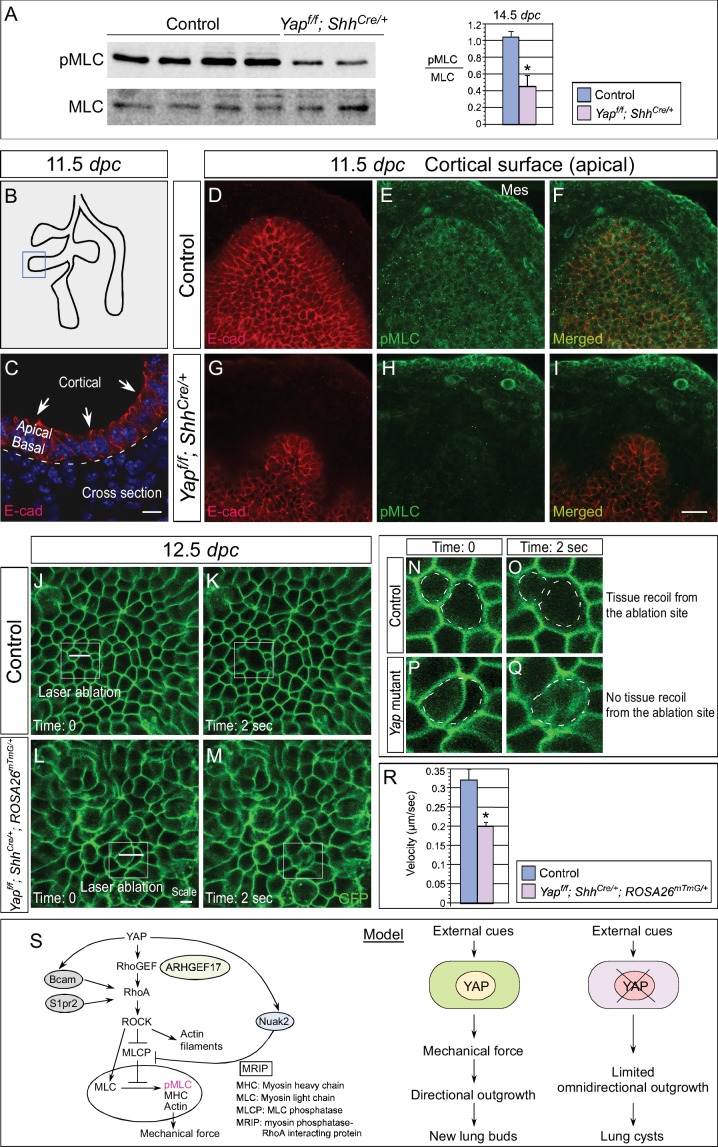
Figure 7. Cortical pMLC is greatly reduced in the epithelium of Yap-deficient lungs and mechanical force production is compromised.
(A) Western blots of lysates derived from control and Yapf/f; ShhCre/+ lungs at 14.5 dpc. Protein levels of phosphorylated myosin light chain (pMLC) were significantly reduced in the absence of Yap. Moreover, the ratio of pMLC to MLC protein levels was also diminished in Yap mutant lungs. (B) Schematic diagram of wild-type lungs at 11.5 dpc. The boxed region indicates areas shown in D–I. (C) Cross-section of a lung bud that illustrates the apical and basal surface and cortical view along the apical surface. (D–I) Whole-mount immunostaining of control and Yapf/f; ShhCre/+ lungs at 11.5 dpc by two-photon microscopy. This enabled visualization of the distribution of pMLC along the cortical surface of epithelial cells located at the apical surface of the lung bud. Lung epithelium was identified by E-cadherin (E-cad). Cortical pMLC was detected in both the epithelium and mesenchyme (mes) of control lungs. By contrast, cortical pMLC could not be detected in the epithelium but retained wild-type levels in the mesenchyme of Yapf/f; ShhCre/+ lungs. (J–M) Laser ablation of control and Yapf/f; ShhCre/+; ROSA26mTmG/+ lungs. Lung epithelial cells were labeled by GFP from the ROSA26mTmG/+ allele induced by ShhCre. Laser ablation of lung epithelial cells by two-photon microscopy (indicated by bars in J,L) triggered recoiling of non-injured neighboring cells in control but not in Yap-deficient lungs (see Videos 1 and 2). Snapshots of the lung epithelium post-ablation were shown. The boxed regions in (J–M) indicate areas shown in (N–Q). (N–Q) The dotted lines in (N,O) demarcate the cell boundary prior to laser ablation in control lungs. Surrounding cells recoiled from the ablation site as indicated by the space between the dotted line and the new position of cells (O). By contrast, no tissue recoil was found in Yap mutant lungs (Q). (R) The velocity of epithelial recoil was measured in control and Yap-deficient lungs after laser ablation. A reduction in velocity was found in the absence of YAP (n ≥ 6 for each group). All values are means ± SEM. (*) p<0.05 (unpaired Student’s t-test). (S) Schematic diagram of signaling cascades that control the production of pMLC and mechanical force. Our model suggests that YAP regulates multiple pathways to regulate pMLC generation. In addition to a YAP-ARHGEF17-RhoA-ROCK pathway, YAP also induces the expression of Bcam, S1pr2 and Nuak2 to enhance pMLC levels. BCAM and S1PR2 activate RhoA, while NUAK2 inhibits MLCP (myosin light chain phosphatase) in a RhoA-independent manner. The employment of a signaling network ensures the production of appropriate amounts of pMLC required for lung branching. Mechanical force production is perturbed in Yap-deficient lungs due to reduced pMLC. This would contribute to defective lung branching and cyst formation. Scale bar = 10 μm for C; 25 μm for D–I.