Figure.
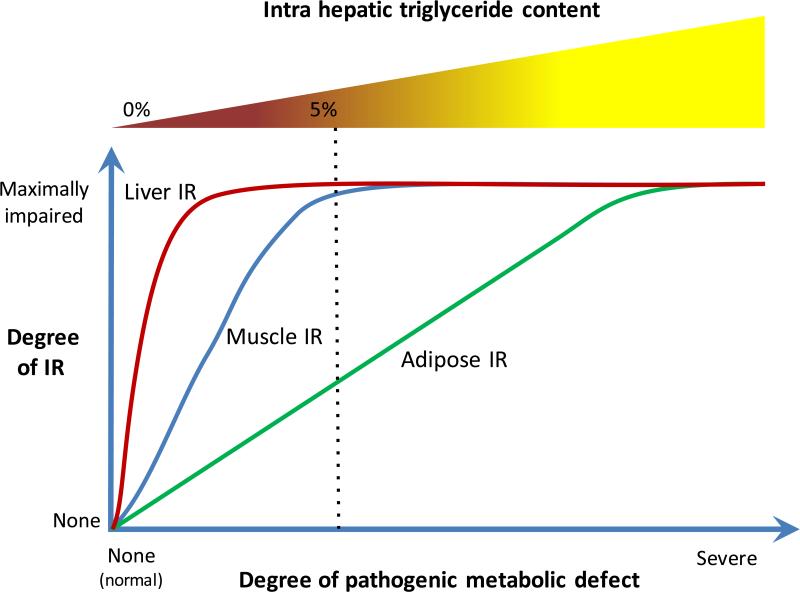
The data provided by Bril et al [reference] from a well-characterized cohort of adults with the full spectrum of NAFLD demonstrates that impairment of insulin responsiveness in the liver (measured by insulin-mediated suppression of endogenous glucose production), muscle (measured by insulin-stimulated glucose uptake), and adipose tissue (measure by insulin-mediated suppression of lipolysis) was markedly different in these three major targets of insulin signaling. One interpretation of their data is that there is an underlying metabolic defect responsible for these changes. Recent studies point to a role of stress activated kinases such as the c-Jun NH2-terminal kinases (JNKs) throughout the body and especially in adipose tissue in mediating these global effects. Using the data from Bril et al, the consequences of this global stress response can be envisioned as shown with liver insulin resistance occurring with minimal provocation, muscle being less easily impaired and adipose tissue the most resistant to being impaired. The accumulation of triglyceride in the liver appears to be a sensitive indicator of adipose insulin resistance and they are correlated over the spectrum of the underlying metabolic abnormality. Interestingly, their data also show that the amount of fat in the liver does not correlate with histological evidence of liver inflammation or hepatocellular injury. Thus the degree of adipose insulin resistance determines the severity of steatosis but it is not directly related to the severity of the indices of NASH.