Abstract
Background
Environmental enteric dysfunction (EED), a condition characterized by small intestine inflammation and abnormal gut permeability, is widespread in children in developing countries and a major cause of growth failure. The pathophysiology of EED remains poorly understood.
Methods
We measured serum metabolites using liquid chromatography-tandem mass spectrometry in 400 children, aged 12–59 months, from rural Malawi. Gut permeability was assessed by the dual-sugar absorption test.
Findings
80.7% of children had EED. Of 677 serum metabolites measured, 21 were negatively associated and 56 were positively associated with gut permeability, using a false discovery rate approach (q < 0.05, p < 0.0095). Increased gut permeability was associated with elevated acylcarnitines, deoxycarnitine, fatty acid β-oxidation intermediates, fatty acid ω-oxidation products, odd-chain fatty acids, trimethylamine-N-oxide, cystathionine, and homocitrulline, and with lower citrulline, ornithine, polyphenol metabolites, hippurate, tryptophan, and indolelactate.
Interpretation
EED is a syndrome characterized by secondary carnitine deficiency, abnormal fatty acid oxidation, alterations in polyphenol and amino acid metabolites, and metabolic dysregulation of sulfur amino acids, tryptophan, and the urea cycle. Future studies are needed to corroborate the presence of secondary carnitine deficiency among children with EED and to understand how these metabolic derangements may negatively affect the growth and development of young children.
Abbreviations: BAIBA, beta-aminoisobutyric acid; EED, environmental enteric dysfunction; LC-MS/MS, liquid chromatography-tandem mass spectrometry; L:M, lactulose to mannitol ratio; MS, mass spectrometry; OCTN2, organic cation/carnitine transporter 2; ROC, receiver operating characteristic curve; RSD, relative standard deviation; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; TMAO, trimethylamine-N-oxide; UPLC-MS/MS, ultra-high performance tandem mass spectrometry
Keywords: Acylcarnitines, Carnitine, Environmental enteric dysfunction, Fatty acid oxidation, Hippurate, Polyphenols, Tryptophan, Urea cycle
Highlights
-
•
Environmental enteric dysfunction (EED) affects millions of children in developing countries.
-
•
We used metabolomics to gain insight into the pathogenesis of EED.
-
•
EED is characterized by secondary carnitine deficiency and abnormal fatty acid oxidation.
-
•
Alterations in sulfur amino acids, tryptophan, urea cycle, and polyphenols were also present.
Environmental enteric dysfunction (EED), an asymptomatic condition of small intestine inflammation and increased gut permeability, is widespread in developing countries and a major cause of growth failure in children. Clinical trials to ameliorate EED have been disappointing. The pathogenesis of EED is poorly understood. We examined the relationship between circulating metabolites and EED in young children in Africa. Children with EED had a serum metabolite profile consistent with secondary carnitine deficiency and impaired β-oxidation of fatty acids. Carnitine is a conditionally essential nutrient primarily found in animal source foods or synthesized endogenously from lysine and methionine. This study suggests that secondary carnitine deficiency may contribute to the altered metabolic profile related to energy balance in young children with EED.
1. Introduction
Environmental enteric dysfunction (EED), an asymptomatic condition characterized by chronic inflammation of the duodenum and jejunum and abnormal gut permeability, is widespread among children under five years of age in developing countries (Trehan et al., 2016). EED is associated with growth failure, oral vaccine failure, impaired absorption of nutrients, and high morbidity and mortality. The pathological changes of EED include epithelial defects of villi, villous shortening, crypt hyperplasia, and infiltration of lymphocytes (Campbell et al., 2003, Kelly et al., 2016). EED typically occurs among young children in the setting of poor sanitation and hygiene, widespread fecal-oral transmission of pathogenic bacteria, viruses, and parasites, and close contact with animals (Watanabe and Petri, 2016). The diet of children at risk for EED is generally of poor quality, being low in animal source foods, essential amino acids, choline, essential fatty acids, and micronutrients (Krebs et al., 2014, Semba et al., 2016a, Semba et al., 2016c, Watanabe and Petri, 2016). Clinical trials to ameliorate EED involving various supplements, antibiotics, anti-inflammatory agents, and improvement in hygiene have been disappointing (Crane et al., 2015, Trehan et al., 2016). To date, interventions have been based upon an incomplete understanding of the pathogenesis of EED.
Some recent studies have aimed to gain insight into the biology and pathogenesis of EED. This area of research includes assessment of human host transcriptome in stool samples (Yu et al., 2015), fecal microbiota through RNA gene sequencing (Ordiz et al., 2016), and selected plasma biomarker evaluation (Guerrant et al., 2016, Kosek et al., 2016). Using a metabolomics approach and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to measure 139 metabolites with a targeted platform (Biocrates, Innsbruck, Austria), we recently identified alterations in serum phosphatidylcholines, sphingomyelins, tryptophan, ornithine, glutamate, taurine, and serotonin in children with EED (Semba et al., 2016b).
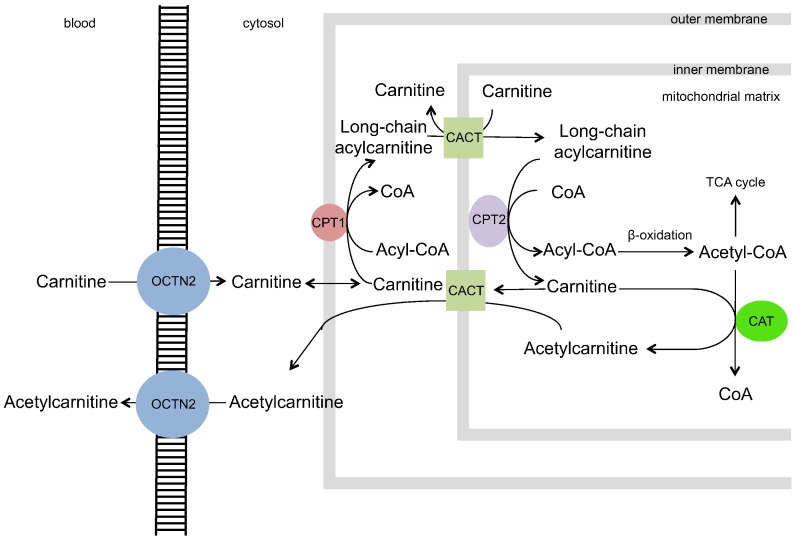
Data from our previous study suggested that secondary carnitine deficiency might occur in children with EED (Semba et al., 2016b). Although serum carnitine itself was not significantly associated with gut permeability, overall in the study population, the median serum carnitine concentrations were ~ 21 μmol/L, which are consistent with secondary carnitine deficiency (Stanley, 2004). These levels are much lower than the reference serum carnitine levels of 40–60 μmol/L described in healthy children and adults (Stanley, 2004, Reuter and Evans, 2012). Carnitine deficiency is classified as primary if due to mutations in the organic cation/carnitine transporter 2 (OCTN2) or secondary if due to inadequate intake of carnitine, decreased biosynthesis of carnitine, genetic defects in enzymes involved in transfer of fatty acids into mitochondria, or defects in enzymes that are required for β-oxidation. Carnitine is essential for the transfer of long-chain fatty acids into the mitochondria for β-oxidation and production of energy in the form of adenosine triphosphate (ATP). In the carnitine shuttle, long-chain fatty acid-CoA in the cytosol exchanges CoA for carnitine by the action of carnitine palmitoyltransferase I at the outer mitochondrial membrane. Acylcarnitine moves into the mitochondrial matrix by facilitated diffusion through a transporter, carnitine-acylcarnitine translocase, on the inner mitochondrial membrane. In the mitochondrial matrix, the acyl group is transferred to mitochondrial coenzyme A by carnitine palmitoyltransferase II. Carnitine is then free to cycle back to the cytosol through the transporter (Fig. 1). Secondary carnitine deficiency is characterized by increases in serum acylcarnitines, fatty acid intermediates associated with blocked β-oxidation, and dicarboxylic acids produced by ω-oxidation of fatty acids (Stanley, 2004, Reuter and Evans, 2012).
Fig. 1.
The carnitine shuttle.
Carnitine enters the cell through active transport by the high affinity carnitine transporter, organic cation transporter novel 2 (OCTN2). Long-chain fatty acid-CoA in the cytosol exchanges CoA for carnitine by the action of carnitine palmitoyltransferase I (CPT1) in the outer mitochondrial membrane. Acylcarnitine moves into the mitochondrial matrix by facilitated diffusion through a transporter, carnitine-acylcarnitine translocase (CACT), on the inner mitochondrial membrane, in exchange for carnitine. In the mitochondrial matrix, the acyl group is transferred to mitochondrial coenzyme A by carnitine palmitoyltransferase II (CPT2). Carnitine is then free to cycle back to the cytosol through the transporter. Carnitine acyltransferase (CAT) removes CoA from acetyl-CoA that is formed from β-oxidation to form acetylcarnitine. Acetylcarnitine can exit the mitochondria via CACT and enter the blood via OCTN2.
We generated a post-hoc hypothesis that EED is associated with secondary carnitine deficiency. To test this hypothesis, we used a wider LC-MS/MS metabolomics platform (Discovery HD4, Metabolon, Durham, NC) to more comprehensively assess metabolites related to carnitine deficiency. This approach also offered the opportunity to expand our discovery into additional pathways that may be dysregulated in children at risk of EED.
2. Methods
2.1. Study Design and Participants
The study design is cross-sectional. The study subjects consisted of 400 children, aged 12–59 months, from six villages (Masika, Makhwira, Mitondo, Mibiza, Chamba, and Mayaka) in rural southern Malawi. Children were enrolled in the study in 2011. Children were eligible for the study if they had no congenital or chronic disease or caretaker-reported diarrhea, or were under treatment for acute malnutrition. Field staff experienced in anthropometry measured weight to the nearest 5 g using a digital scale (Seca 344, Chino, CA) and height to the nearest 0.1 cm using a rigid height board (Seca 417). Gut integrity was assessed using the dual sugar absorption test, as described in detail elsewhere (Weisz et al., 2012). The urinary lactulose:mannitol (L:M) ratio, as derived from the dual sugar absorption test, was used as the measure of gut integrity (Denno et al., 2014). Children with an L:M ratio ≥ 0.15 were defined as having EED (Lord and Bralley, 2008). Lactulose and mannitol were measured using high-performance liquid chromatography (Weisz et al., 2012). Briefly, children arrived at the clinic site at 6 am without having eaten any food for the day yet. Children ingested a solution of 1 g mannitol and 5 g lactulose completely dissolved in water while being observed not to have any spitting or spilling. A transparent urine collection bag was then affixed with additional adhesive, and clothing removed so the urine bag could be continuously observed by the clinical staff. Immediately upon note of any urine in the collection bag, the urine was retrieved and a clean bag applied. A complete 4 h collection for every child was obtained, which meant that every child passed fresh urine at least 4 h after ingesting the sugars. Urine passage was facilitated by offering water to drink about 3 h after ingestion of sugars. If urine was spilled or sugar solutions not ingested the child was asked to return for a repeat test in 48 h. Malawian research nurses obtained written and oral informed consent from each child's caretaker before enrollment in the study. Community level consent for the study also was obtained from the village chief and local health officials. The study protocol was approved by the College of Medicine Research Ethics Committee of the University of Malawi, the Human Research Protection Office of Washington University in St. Louis, and the Johns Hopkins School of Medicine Institutional Review Board. The study conforms to the standards indicated by the Declaration of Helsinki. The methods were carried out in accordance with the approved guidelines.
2.2. Measurement of Serum Metabolites
Venous blood was drawn by study nurses and doctors. Serum samples were processed, aliquoted, and snap frozen in liquid nitrogen in cryovials within 4 h of blood drawing. Cryovials were transferred to storage at − 80 °C until time of analysis. All experimental samples were prepared and analyzed in a masked fashion. Samples were sent in a random order and were prepared at Metabolon, Inc. (Durham, NC) as described elsewhere (Evans et al., 2009). The staff at Metabolon had no access to the study database. In brief, for quality control, recovery standards were added prior to the first step of the extraction process. Proteins were precipitated with methanol under vigorous shaking for 2 min using a GenoGrinder 2000 (Glen Mills, Clifton, NJ) followed by centrifugation. The resulting extract was divided into five fractions: (i) early and (ii) late eluting compounds for analysis by ultra-high performance LC-MS/MS (UPLC-MS/MS) using positive ionization, (iii) for analysis by UPLC-MS/MS using negative ionization, (iv) for analysis using a UPLC-MS/MS polar platform with negative ionization, and (v) a sample reserved for backup if needed.
Three types of controls were analyzed concomitantly with the experimental samples: (i) samples generated from a pool of human plasma that has been extensively characterized by Metabolon, (ii) extracted water samples serving as process blanks, and (iii) a cocktail of standards spiked into every analyzed sample to allow monitoring of instrument performance. Instrument variability was determined by calculating the median relative standard deviation (RSD) for the standards that were added to each sample prior to injection into the mass spectrometers (median RSD typically = 4–6%; n ≥ 30 standards). Overall process variability was determined by calculating the median RSD for all endogenous metabolites (i.e., non-instrument standards) present in 100% of the pooled human plasma samples (median RSD = 10–14%; n = several hundred metabolites). Experimental samples and controls were randomized across the experimental runs.
Sample extracts were analyzed using a standardized chromatographic UPLC-MS/MS method (Evans et al., 2014). All columns and solvents were obtained from a single manufacturer's lot for the sample analysis of this study. For each sample, vacuum-dried samples were dissolved in injection solvent containing eight or more injection standards at fixed concentrations, depending on the platform. The internal standards were used to ensure consistency of injections and chromatography. Instruments were tuned and calibrated for mass resolution and mass accuracy daily.
The UPLC-MS/MS platform consisted of an Acquity UPLC (Waters Corp., Milford, MA) and a Q-Exactive (Thermo Scientific, Framingham, MA) mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and an Orbitrap mass analyzer operated at 35,000 mass resolution. The sample extract was dried and then reconstituted in acidic or basic LC-compatible solvents, each of which contained 8 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic, positive ion-optimized conditions. Another aliquot was analyzed using basic, negative ion-optimized conditions. Two independent injections were done using separate dedicated columns (Waters UPLC BEH C18–2.1 × 100 mm, 1.7 μm). Extracts reconstituted in acidic conditions were gradient-eluted using water and methanol containing 0.1% formic acid, while the basic extracts, which also used water/methanol, contained 6.5 mM ammonium bicarbonate. A third aliquot was analyzed via negative ionization following elution from a HILIC column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 μm) using a gradient consisting of water and acetonitrile with 10 mM ammonium formate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion. The scan range was from 80 to 1000 m/z.
Metabolites were identified by an automated comparison of the ion features in the experimental samples against a reference library of chemical standard entries. The entries included molecular weight (m/z), retention time, preferred adducts, in-source fragment, and associated MS spectra. Entries have been curated by visual inspection for quality control using software developed at Metabolon (DeHaven et al., 2010). The identification of known metabolites is based upon comparison to metabolomic library entries of purified standards. Commercially available purified standard compounds have been acquired and registered into LIMS for determination of their detectable characteristics. Peaks were quantified using area-under-the-curve. Raw area counts for each metabolite in each sample were normalized to correct for variation resulting from instrument inter-day tuning differences by the median value for each run-day, therefore, setting the medians to 1.0 for each run. This normalization preserved the variation between samples but allowed metabolites of widely different raw peak areas to be compared on a similar graphical scale. Missing values were imputed with the observed minimum after normalization.
2.3. Statistical Analysis
The distributions of serum metabolites and the L:M ratio were examined in exploratory data analyses using histograms and boxplots. The primary analysis of this study is based upon gut permeability, as represented by the L:M ratio as a continuous variable. Spearman correlations of each metabolite with the L:M ratio were estimated with adjustment for age, gender, and village. A false discovery rate approach was used to correct for multiple testing. Q-values were computed using the bootstrap method (Storey, 2002, Storey et al., 2004, Rachakonda et al., 2014). This analysis was carried out using the pcor.test() function in the ppcor package in R software version 3.2.0. No a priori power analysis was conducted for this study. The sample size of 400 represented all the serum samples collected in six villages in 2011. Serum metabolites in children with and without EED were compared using Wilcoxon rank-sum test. t-tests and chi-square tests were used to compare continuous and categorical variables, respectively, between children with and without EED. Classification modeling using eight machine-learning algorithms was used to examine the ability of the 77 metabolites that were significantly correlated with L:M ratio to classify EED status (L:M ≥ 0.15 versus L:M < 0.15). We used the Super Learner, an ensembling approach to machine learning, (van der Laan et al., 2007) to fit, internally cross-validate, and combine the results into a single classifier. Eight different classifiers were trained on a subset of the data as described in Supplementary Appendix 1. A weighted-average of the estimates was then computed to minimize mean-square error. The weights themselves were estimated via leave 10% out internal cross-validation. This method has been described in greater detail elsewhere (Semba et al., 2016b). Details of Super Learner, including computation of estimates, cross-validation, and metrics to assess the discrimination and calibration, have been described in detail elsewhere (Semba et al., 2016b). Classification modeling was carried out using the SuperLearner() and CV·SuperLearner() functions in the Super Learner package in R software version 3.2.0.
3. Results
The demographic characteristics of the 400 children are shown in Table 1. Three hundred twenty-three (80.7%) children had EED. Children with EED were significantly younger, more likely to have a living father, reside in a home without a metal roof (an indicator of lower socioeconomic status), and less likely to use water from a clean source than children without EED. There were significant differences in prevalence of EED among the six villages. There were no significant differences in gender, anthropometric measurements, or number of siblings between children with and without EED.
Table 1.
Characteristics of the study population.
| Characteristica | No EEDb (n = 77) | EEDb (n = 323) |
pc | |
|---|---|---|---|---|
| Age, months | 38.0 ± 10.2 | 32.9 ± 12.1 | 0.0007 | |
| Female, % | 35 (45%) | 163 (50%) | 0.43 | |
| Weight-for-height Z-score | 0.3 ± 1.0 | 0.1 ± 1.0 | 0.29 | |
| Height-for-age Z-score | − 2.5 ± 1.3 | − 2.3 ± 1.3 | 0.25 | |
| Stunted, % | 52 (68%) | 198 (61%) | 0.31 | |
| Primary caretaker is mother, % | 73 (95%) | 308 (95%) | 0.84 | |
| Father is alive, % | 69 (70%) | 315 (98%) | 0.001 | |
| Siblings, n | 3.7 ± 1.8 | 3.8 ± 1.7 | 0.64 | |
| Individuals that sleep in same room as child, n | 3.2 ± 1.6 | 3.3 ± 1.4 | 0.78 | |
| Home with a metal roof, % | 23 (30%) | 52 (16%) | 0.005 | |
| Family owns bicycle, % | 44 (57%) | 200 (62%) | 0.44 | |
| Animals sleep in house, % | 34 (44%) | 110 (34%) | 0.10 | |
| Water from a clean source, % | 64 (83%) | 208 (64%) | 0.002 | |
| Child uses pit latrine, % | 56 (73%) | 256 (79%) | 0.21 | |
| Village, % | Chamba | 7 (9%) | 38 (12%) | < 0.001 |
| Makwhira | 5 (6%) | 22 (7%) | ||
| Masika | 11 (14%) | 145 (45%) | ||
| Mayaka | 27 (35%) | 69 (21%) | ||
| Mbiza | 20 (26%) | 40 (12%) | ||
| Mitondo | 7 (9%) | 9 (3%) | ||
Means (SD) or %.
No EED (L:M < 0.15), EED (L:M ≥ 0.15).
Students t-test for continuous variables or chi-square test for categorical variables.
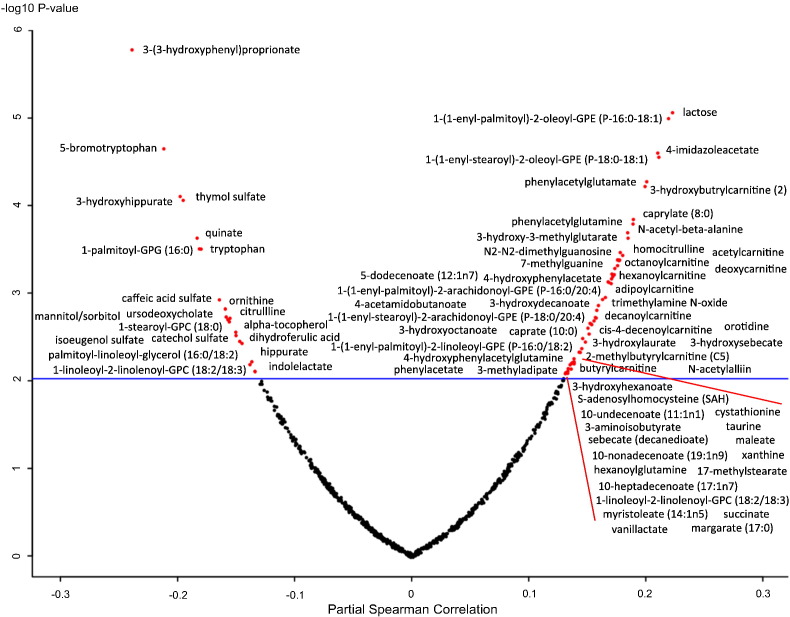
The relationship of gut permeability, as assessed by the L:M ratio, with serum metabolites is summarized in a volcano plot in Fig. 2. There were 77 metabolites that were significantly associated with gut permeability. Twenty-one metabolites were negatively associated with gut permeability, including six metabolites related to dietary polyphenols, citrulline, ornithine, tryptophan, and indolelactate (Table 2). Fifty-six metabolites were positively associated with gut permeability, including 9 acylcarnitines, deoxycarnitine, 3 intermediates of β-oxidation of fatty acids, 4 metabolites from ω-oxidation of fatty acids, 4 odd-chain fatty acids, trimethylamine-N-oxide, cystathionine, and homocitrulline (Table 3).
Fig. 2.
Volcano plot showing relationship of partial Spearman correlations between gut permeability, as measured by the L:M ratio, and serum metabolites, adjusted for age, sex, and village.
Horizontal line indicates significance at p-value of 0.0096, which corresponds to a q-value < 0.05.
Table 2.
Serum metabolites negatively associated with gut permeability (L:M ratio).
| Metabolite | Description |
|---|---|
| 3-(3-hydroxyphenyl)proprionate | major metabolite of caffeic acid, a dietary polyphenol |
| 5-bromotryptophan | non-proteinogenic α-amino acid |
| thymol sulfate | metabolite of thymol from plants, fat-soluble |
| 3-hydroxyhippurate | dietary polyphenol |
| quinate | dietary polyphenol |
| 1-palmitoyl-GPG (16:0) | glycerophospholipid |
| Tryptophan | essential amino acid |
| Caffeic acid sulfate | dietary polyphenol |
| Ornithine | non-proteinogenic amino acid in urea cycle |
| Ursodeoxycholate | bile acid |
| Citrulline | α-amino acid and key intermediate in urea cycle |
| 1-stearoyl-GPC (18:0) | glycerophosphocholine |
| Alpha-tocopherol | vitamin E, fat-soluble |
| Sorbitol/mannitol | isomers; sugar |
| Catechol sulfate | phenylsulfate |
| Isoeugenol sulfate | phenylpropene from plants, fat-soluble |
| Dihydroferulic acid | dietary polyphenol |
| Hippurate | metabolite from dietary polyphenol degradation |
| Indolelactate | tryptophan metabolite |
| Palmitoyl-linoleoyl-glycerol (16:0/18:2) | triacylglycerol |
| 1-linoleoyl-2-linolenoyl-GPC (18:2/18:3) | glycerophosphocholine |
Table 3.
Serum metabolites positively associated with gut permeability (L:M ratio).
| Metabolite | Description |
|---|---|
| Lactose | disaccharide in human milk |
| 1-(1-enyl-palmitoyl)-2-oleoyl-glycerophosphoethanolamine (P-16:0/18:1) | glycerophosphoethanolamine |
| 4-imidazoleacetate | histidine metabolite |
| 1-(1-enyl-stearoyl)-2-oleoyl-glycerophosphoethanolamine (P-18:0/18:1) | glycerophosphoethanolamine |
| Phenylacetylglutamate | can be formed from phenylacetylglutamine |
| 3-hydroxybutrylcarnitine | acylcarnitine, C:4-OH |
| Caprylate (8:0) | 8 carbon saturated fatty acid, in human milk |
| Phenylacetylglutamine | formed by the conjugation of phenylacetate and glutamine |
| N-acetyl-beta-alanine | beta amino acid |
| 3-hydroxy-3-methylglutarate | metabolite related to leucine degradation and ketogenesis |
| N2-N2-dimethylguanosine | primary degradation product of tRNA |
| Homocitrulline | ornithine metabolite |
| Acetylcarnitine | acylcarnitine, C:2 |
| 7-methylguanine | metabolite of DNA methylation and depurination |
| Octanoylcarnitine | acylcarnitine, C:8 |
| Hexanoylcarnitine | acylcarnitine, C:6 |
| 5-dodecenoate (12:1n7) | intermediate in β-oxidation of unsaturated fatty acids |
| Deoxycarnitine | metabolic precursor of carnitine |
| 4-hydroxyphenylacetate | tyrosine metabolite |
| 1-(1-enyl-palmitoyl)-2-archidonoyl-GPE (P-16:0/20:4) | glycerophosphoethanolamine |
| 4-acetamidobutanoate | member of gamma amino acids and derivatives |
| 3-hydroxydecanoate | intermediate in β-oxidation of fatty acids |
| Adipoylcarnitine | acylcarnitine, C:6-DC |
| Trimethylamine N-oxide | oxidation product of trimethylamine |
| Decanoylcarnitine | acylcarnitine, C:10 |
| Caprate (10:0) | 10 carbon medium chain saturated fatty acid |
| cis-4-decenoylcarnitine | acylcarnitine, C10:1 |
| 3-hydroxyoctanoate | dicarboxylic acid derived from ω-oxidation of fatty acids |
| 4-hydroxyphenylacetylglutamine | formed from conjugation of phenylacetate and glutamine |
| 3-hydroxylaurate | intermediate in β-oxidation of fatty acids |
| 1-(1-enyl-stearoyl)-2-archidonoyl-GPE (P-18:0/20:4) | glycerophosphoethanolamine |
| 3-hydroxysebacate | dicarboxylic acid derived from ω-oxidation of fatty acids |
| Orotidine | intermediate in pyrimidine nucleotide biosynthesis |
| 2-methylbutyrylcarnitine (C5) | acylcarnitine, 2-M-C4:0 |
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPE (P-16:0/18:2) | glycerophosphoethanolamine |
| 3-methyladipate | metabolite from ω-oxidation of phytanic acid |
| Phenylacetate | fatty acid metabolite of phenylalanine |
| Butyrylcarnitine | acylcarnitine, C:4 |
| N-acetylalliin | alanine derivative |
| 3-hydroxyhexanoate | hydroxyl fatty acid |
| S-adenosylhomocysteine (SAH) | product of methylation reactions of S-adenosylmethionine |
| 10-undecenoate (11:1n1) | odd-chain fatty acid |
| cystathionine | formed from transsulfuration of homocysteine |
| 3-aminoisobutyrate | formed from catabolism of thymine |
| Taurine | derived from cysteine, major component of bile |
| Sebacate (decanedioate) | dicarboxylic acid derived from ω-oxidation of fatty acids |
| Maleate | dicarboxylic acid |
| Xanthine | product of purine degradation |
| 10-nonadecenoate (19:1n9) | odd-chain fatty acid |
| Hexanoylglutamine | α-amino acid conjugated with hexanoyl group |
| 17-methylstearate | medium chain iso-fatty acid |
| 10-heptadecenoate (17:1n7) | odd-chain fatty acid |
| Myristoleate (14:1n5) | Ω-5 fatty acid |
| Vanillactate | product of catecholamine degradation |
| Margarate (17:0) | odd-chain fatty acid |
| Succinate | intermediate in the citric acid cycle |
The age-, sex-, and village-adjusted Spearman correlations between gut permeability and serum metabolites are presented in Supplementary Table 1. Serotonin was positively associated with gut permeability but was of marginal significance (p = 0.0098, q = 0.050007). Serum metabolites in children with and without EED were compared in Supplementary Table 2.
When multiple machine-learning classification algorithms were used to assess the ability of the 77 metabolites significantly correlated with the L:M ratio to classify EED status (EED vs. no EED), six algorithms had positive weights: LASSO (Friedman et al., 2010), ridge regression (Friedman et al., 2010), generalized boosted models (Friedman, 2001), random forests (Breiman, 2001), multivariate adaptive regression splines (Friedman, 1991) implemented using the polymars() function in R software version 3.20, and Bayes generalized linear models. A receiver operating characteristic curve (ROC) is shown in Supplementary Appendix Fig. 1. (Supplementary Appendix Fig. 1). The Super Learner cross-validated area under the ROC curve was 0.710 (95% confidence interval 0.643, 0.777). The final cross-validated Super Learner model was well calibrated showing little evidence of lack of fit (Hosmer-Lemeshow p-value = 0.91) (Supplementary Appendix Table 1). These findings show that the 77 serum metabolites that were significantly associated with gut permeability have a modest ability to discriminate between children with and without EED.
4. Discussion
The present study shows that increased gut permeability is associated with elevated serum concentrations of nine acylcarnitines, three intermediate metabolites associated with blocked β-oxidation of fatty acids, and four metabolites related to upregulation of ω-oxidation of fatty acids, a serum metabolite profile that is consistent with secondary carnitine deficiency and defective fatty acid oxidation (Stanley, 2004, Tein, 2013). Carnitine is a conditionally essential nutrient that plays a vital role in fatty acid metabolism and energy production. This study shows that secondary carnitine deficiency is associated with EED.
In healthy humans consuming a normal diet, ~ 75% of carnitine is from food and the remaining ~ 25% is synthesized by the body (Kendler, 1986). The richest dietary sources of carnitine are animal source foods, such as red meat, chicken, fish, and dairy products. Plant foods only contain negligible amounts of carnitine (Demarquoy et al., 2004). Carnitine is synthesized in the body from two essential amino acids, lysine and methionine. Lysine provides the carbon backbone and nitrogen atom of carnitine, while methionine provides the methyl groups. Cofactors necessary for this synthesis include niacin, pyridoxine, ascorbic acid, and ferrous iron. In many parts of the developing world, including rural Malawi, animal source foods are rarely consumed (Dror and Allen, 2011), and lysine is a limiting essential amino acid in the maize-based diet (Nuss and Tanumihardjo, 2011). A limitation of dietary lysine could theoretically increase the risk of carnitine deficiency, however serum lysine was not significantly associated with gut permeability. Experimental animal models show that lysine deficiency leads to impaired carnitine synthesis and fatty acid abnormalities (Tanphaichitr and Broquist, 1973, Khan and Bamji, 1979). In addition to its role in fatty acid metabolism, carnitine may play an important role in normal gut function. Enterocytes synthesize carnitine from lysine and methionine (Shekhawat et al., 2013). Mice with loss of functional mutations in OCTN2 show villous atrophy and inflammation in the small intestine (Shekhawat et al., 2007, Sonne et al., 2012).
The most common causes of secondary carnitine deficiency are inborn errors of metabolism (Onkenhout et al., 1995, Mamedov et al., 2015) and acquired deficiency. Causes of acquired deficiency include poor dietary intake of carnitine, impaired absorption of dietary carnitine, decreased endogenous synthesis of carnitine, and increased renal losses of carnitine (Crill and Helms, 2007). Secondary carnitine deficiency has been reported in children with kwashiorkor and marasmus (Alp et al., 1999), celiac disease (Lerner et al., 1993), and lysinuric protein intolerance (Tanner et al., 2008). Secondary carnitine deficiency also occurs in children receiving renal or peritoneal dialysis (Sgambat and Moudgil, 2016, Naseri et al., 2016) or soy-protein-based formula lacking carnitine (Winter et al., 1987, Olson et al., 1989).
Plasma carnitine is actively transported across the plasma membrane by OCTN2. As noted previously, the carnitine shuttle is essential to deliver long-chain fatty acids into the mitochondria for β-oxidation and energy production. Short- and medium-chain fatty acids do not require carnitine transport into the mitochondria. Carnitine is involved in the oxidation of medium-chain fatty acids in the cytosol, under control of acetyltransferase for short-chain acyl groups and octanoyltransferase for medium-chain acyl groups. Carnitine also plays an important role in maintaining a free CoA pool in the cytosol and within mitochondria, as shown in Fig. 1. The transport of acylcarnitines out of the mitochondria maintains the mitochondrial pool of free CoA and protects mitochondrial against toxic accumulation of acyl CoA compounds that could inhibit enzymatic activity and alter ATP production (Crill and Helms, 2007).
With secondary carnitine deficiency, acylcarnitines accumulate within cells and the circulation. The long-chain acylcarnitines that accumulate with impaired β-oxidation within the cell are potent inhibitors of OCTN2 (Stanley, 2004). In the kidney, carnitine is freely filtered at the glomerulus, but 95% of filtered carnitine is reabsorbed by the proximal tubule via OCTN2 and returned to the circulation (Reuter and Evans, 2012). Carnitine is preferentially reabsorbed by OCTN2 over acylcarnitines, thus, the renal clearance of acylcarnitines may be 10–20 times higher than free carnitine (Carroll et al., 1981). As a consequence, in secondary carnitine deficiency, elevated circulating acylcarnitines are excreted in the urine and can contribute further to depletion of the carnitine pool of the body.
In children with increased gut permeability in the present study, elevated serum levels of intermediates in the β-oxidation of fatty acids, such as 5-dodecenoate, 3-hydroxydecanoate, and 3-hydroxylaurate suggest that β-oxidation of fatty acids is blocked. Elevated levels of circulating 5-dodecenoate have been described in children with medium-chain acyl-CoA dehydrogenase deficiency and impaired β-oxidation (Onkenhout et al., 1995). Children with defective β-oxidation due to long-chain 3-hydroxyacylcoenzyme A dehydrogenase deficiency have increased circulating 3-hydroxydecanoate concentrations (Dorland et al., 1991). Serum 3-hydroxylaurate (3-hydroxydodecanoic acid) levels are elevated in children with long-chain hydroxyacyl CoA dehydrogenase deficiency and defective β-oxidation of fatty acids (Chickos et al., 2002).
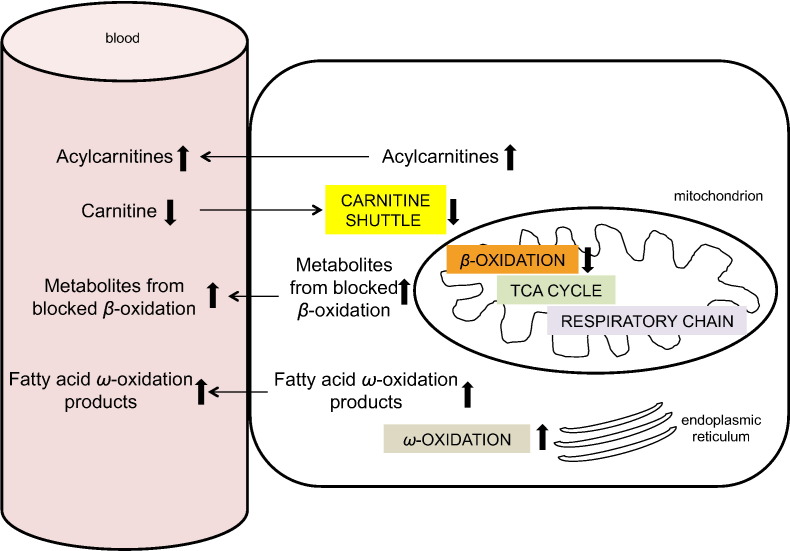
When β-oxidation of fatty acids is blocked, there is an increase in ω-oxidation of fatty acids. ω-oxidation is normally a minor subsidiary path in endoplasmic reticulum that provides succinyl-CoA for the citric acid cycle and for gluconeogenesis (Miura, 2013). In the present study, children with EED had elevated dicarboxylic acids produced by ω-oxidation: sebacate (decanedioate), 3-hydroxysebacate, and 3-hydroxyoctanoate. Increased urinary excretion of sebacate and other dicarboxylic acids produced by ω-oxidation has been described in infants with inadequate carnitine intake (Olson et al., 1989). Serum 3-methyladipate, a metabolite of the ω-oxidation pathway of phytanic acid, a branched chain fatty acid (Wierzbicki et al., 2003), was also elevated in children with EED. The abnormalities in carnitine metabolism found in the present study are summarized in Fig. 3.
Fig. 3.
Serum metabolite profile in secondary carnitine deficiency.
With low carnitine and a defective carnitine shuttle, β-oxidation is blocked and intermediates of blocked β-oxidation accumulate. To compensate for blocked β-oxidation, ω-oxidation, which is usually a minor fatty acid oxidation pathway in endoplasmic reticulum, is upregulated with accumulation of ω-oxidation products.
Children with increased gut permeability had higher serum concentrations of four odd-chain fatty acids. Less is known about odd-chain fatty acids compared with even-chain fatty acids, since they represent < 1% of total fatty acid plasma concentrations in humans (Jenkins et al., 2015). The etiology of the increase in odd-chain fatty acids in children with EED is unclear. It has been proposed that odd-chain fatty acids can be metabolized by α-oxidation, which would involve the activation and subsequent hydroxylation of the α-carbon followed by removal of the terminal carboxyl group (Jenkins et al., 2015). Since β-oxidation may be blocked in EED, a possible mechanism for an endogenous rise in odd-chain fatty acids may be an increase in α-oxidation.
Increased gut permeability was associated with elevated serum 3-hydroxy-3-methylutarate, which has also been described in 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency, an autosomal recessive disease characterized by secondary carnitine deficiency, defective β-oxidation of fatty acids, and impaired leucine catabolism and ketogenesis (da Rosa et al., 2016, Puisac et al., 2010). Children with increased gut permeability also had higher serum 3-aminoisobutyrate (beta-aminoisobutyric acid, BAIBA), a non-protein β-amino acid generated by the catabolism of valine. BAIBA is a small molecule myokine that induces β-oxidation of fatty acids in hepatocytes (Roberts et al., 2014). Whether the elevation of BAIBA is a compensatory response related to blocked β-oxidation in EED could be studied in the future. Dietary carnitine can be converted to trimethylamine-N-oxide (TMAO) by the gut microbiome (Koeth et al., 2013). Children with increased gut permeability also had higher serum TMAO concentrations.
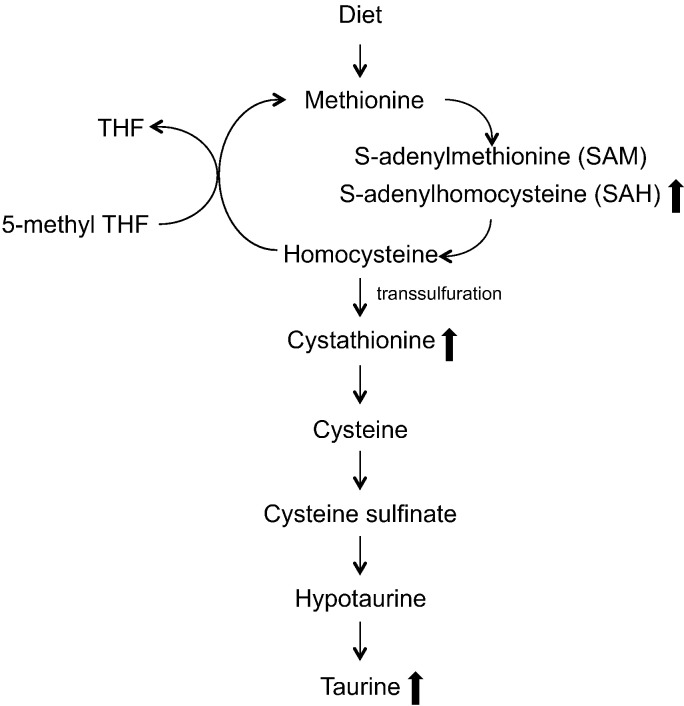
The present study supports the idea that EED is associated with abnormal metabolism of sulfur amino acids and upregulation of the transsulfuration pathway towards production of cystathionine and taurine (Fig. 4). Children with increased gut permeability had elevated serum S-adenosylhomocysteine (SAH), the immediate precursor to homocysteine, and cystathionine, a metabolite produced from the transsulfuration of homocysteine. Bickler and colleagues hypothesized that increased oxidative stress in EED could drive methionine metabolism towards cystathionine synthesis, limiting the sulfur amino acids from participating in protein synthesis (Bickler et al., 2011). Cystathionine is metabolized to taurine through intermediate metabolites cysteine, cysteine, sulphinate, and hypotaurine. Children with EED also had elevated serum taurine. Enterocytes are among the most rapidly proliferating cells in the body, thus reduced availability of sulfur amino acids for synthesis of proteins could potentially contribute to failure of the intestinal barrier (Bickler et al., 2011). Increased transsulfuration of homocysteine could potentially affect the availability of methionine for carnitine synthesis. Methionine provides the methyl group for carnitine synthesis via S-adenylmethionine (SAM) (Fig. 4).
Fig. 4.
Metabolism of sulfur amino acids.
Methionine is metabolized through transmethylation produces homocysteine. Homocysteine is remethylated through 5-methyl tetrahydrofolate (THF) to produce methionine. During inflammation, homocysteine undergoes transsulfuration to produce cystathione and eventually taurine. Children with increased gut permeability had elevated serum cystathione and taurine, suggesting an increase in the transsulfuration pathway.
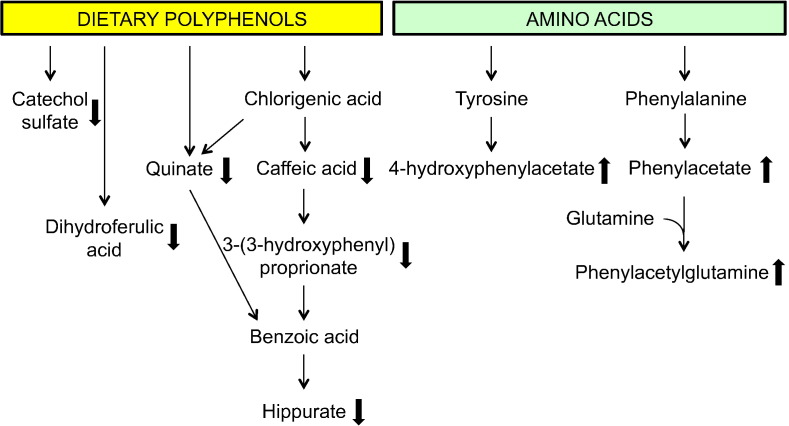
The present study is suggestive that children with EED may have altered metabolism associated with an abnormal gut microbiome, including metabolism of dietary polyphenols, phenylalanine, and tyrosine (Fig. 5). Children with EED had decreased serum hippurate, 3-hydroxyhippurate, and dietary polyphenolic compounds that are metabolic intermediates in the pathway to hippurate (Lees et al., 2013), including caffeic acid sulfate, 3-(3-hydroxyphenyl) proprionate, and quinate. Hippurate is strongly associated with diet and the gut microbiome (Lees et al., 2013). Increased gut permeability was also associated with lower serum levels of dihydroferulic acid, a polyphenol found in tea and fruit (Pimpão et al., 2015). Tea is a common beverage consumed by young children in rural Malawi. Decreased serum catechol sulfate, a phenolic sulfate derived from the catabolism of polyphenols by the gut microbiome (Pimpão et al., 2015), was also associated with increased gut permeability.
Fig. 5.
Abnormalities in serum metabolites known to be associated with abnormal gut microbiome.
Metabolites known to be related to dietary polyphenols and an abnormal gut microbiome were decreased in children with increased gut permeability. Metabolites known to be associated with amino acid fermentation by an abnormal gut microbiome were increased in children with increased gut permeability.
Serum phenylacetylglutamine and 4-hydroxyphenylacetylglutamine, which provide a major route of excretion of excess nitrogen from the body, were elevated in children with EED. Phenylacetylglutamine is formed from the conjugation of phenylacetate (from the gut microbiome or host) and glutamine (mainly generated from the Krebs cycle from α-ketoglutarate). Phenylacetate is primarily produced by the gut microbiome by the decarboxylation of phenylalanine in unabsorbed protein residues (Seakins, 1971). Children with EED also had elevated serum phenylacetate. Elevated urinary phenylacetylglutamine has been described in children with inflammatory bowel disease (Martin et al., 2016). Elevated serum phenylacetylglutamine was an independent risk factor for mortality and cardiovascular disease in nearly 500 patients with chronic kidney disease (Poesen et al., 2016). It is unclear whether phenylacetylglutamine has direct toxic effects or is a marker for altered colonic microbial metabolism and/or renal dysfunction (Poesen et al., 2016). Elevated serum 4-hydroxyphenylacetate was found in children with EED in the present study. 4-hydroxyphenylacetate, one of the most abundant phenylpropanoid-derived compounds found in human fecal samples, is derived from fermentation of tyrosine by the gut microbiome (Russell et al., 2013).
Our previous studies (Semba et al., 2016b) showed that children with increased gut permeability have lower circulating tryptophan concentrations and elevated serotonin concentrations, which was corroborated in the present study using a different metabolomics platform. A new finding is that increased gut permeability was also associated with low indolelactate, which is a metabolite in tryptophan catabolism through a series of indoles. The present study also corroborates the relationship between increased gut permeability and low citrulline levels, an observation also made in a study of children from Peru and Tanzania (Kosek et al., 2016). We also showed in the previous (Semba et al., 2016b) and present study that children with increased gut permeability had low serum ornithine, a component of the urea cycle. An adequate supply of citrulline and ornithine is required for normal function of the urea cycle. A new finding is that children with increased gut permeability had elevated homocitrulline levels. In the absence of ornithine from the urea cycle, mitochondrial carbamoyl phosphate increases and can generate homocitrulline from lysine via ornithine transcarbamylase.
Using the Metabolon platform in the present study, we were unable to corroborate some findings that were significant in the previous study that used the Biocrates metabolomics platform, such as a positive association between serum glutamate and gut permeability. In addition, although the present study showed that carnitine and nine acylcarnitines were positively associated with gut permeability, the previous study using the Biocrates platform did not show a significant association between carnitine and acylcarnitines with gut permeability. The Biocrates p180 kit has a semi-quantitative method that measures 39 specific acylcarnitines, however, in our study only seven acylcarnitines were above the limit of detection, based on our settings of only using metabolites that were detected in > 80% of the sample population (Semba et al., 2016b). This underscores the importance of carrying out LC-MS/MS assays that provide absolute quantification specifically for carnitine and acylcarnitines in the future (Giesbertz et al., 2015, Minkler et al., 2015).
The low serum mannitol/sorbitol (these two sugars are isomers that cannot be distinguished in the MS assay) concentrations and elevated serum lactose levels provide corroborative evidence for reduced small intestinal surface area and increased gut permeability, respectively, in children with EED. Mannitol and sorbitol are monosaccharides that readily cross the intact gut barrier. Serum mannitol concentrations are reduced proportional to the intestinal surface (Sigalet et al., 2000). Lactose is a disaccharide that, like lactulose, does not cross a normal intact intestinal barrier. Serum lactose was positively correlated with gut permeability. EED is associated with malabsorption of fats. In the present study, low serum concentrations of three fat-soluble substances, α-tocopherol (vitamin E), thymol (a monoterpene phenol found in oils from plants), and isoeugenol sulfate (a substance from plants), are consistent with fat malabsorption in EED.
This study is limited in that the metabolomic abnormalities described in children in Malawi may not be generalizable to other populations due to differences in diet, environment, socioeconomic factors, and other variables. The Metabolon HD4 platform provides wide coverage of many metabolic pathways, but the serum metabolite measurements are semi-quantitative. This discovery platform can foster the development of targeted LC-MS/MS assays that provide absolute quantification using deuterated or stable isotope-labeled standards. Such targeted assays could be used for absolute quantification of specific metabolites such as acylcarnitines and dicarboxylic acids in the future. Although the present study shows abnormalities in metabolites that are known to be associated with altered gut microflora, the gut microbiome was not assessed in the present study. However, a previous studies show that EED is associated with alterations in the gut microbiome (Yu et al., 2015, Ordiz et al., 2016). Another limitation is that EED remains a poorly defined disease state that is ideally assessed by biopsy of the small intestine and endoscopy. The dual sugar absorption test is commonly used for diagnosis but has potential for misclassification (Denno et al., 2014). Future studies are needed in children with EED to examine the relationship between serum metabolites and the gut microbiome and between serum metabolites and vaccine failure. Also, studies using a longitudinal design could examine the relationship between serum metabolites and growth faltering. The strengths of this study are the large sample size, the use of a highly advanced metabolomics platform, and a community-based study design in a setting where EED is a major cause of morbidity.
The present study suggests that secondary carnitine deficiency could be widespread among children in developing countries where intake of animal source foods is low and lysine is a limiting essential amino acid in the diet. There are millions of children in developing countries where EED is common who subsist on maize, cassava, sorghum, or rice, and have poor intake of meat, eggs, and dairy products. Secondary carnitine deficiency was associated with increased gut permeability in this epidemiological study. This cross-sectional association does not show causality or definitive proof that EED is related to carnitine. Controlled studies of carnitine supplementation and stable isotope studies will be needed in the future to provide stronger evidence for a role of carnitine among children with EED in developing countries.
The following are the supplementary data related to this article.
Classification modeling using Super Learner algorithm
Spearman correlations of serum metabolites with L:M ratio
Serum metabolites in children with and without EED
Conflicts of Interest
The authors have no conflicts of interest.
Author Contributions
RDS, MS, lT, KK, LF, RM, MM were responsible for the study design, statistical analyses, data interpretation, and writing the manuscript. XL, MlO, and MS conducted the data analysis. lT, KMM, and MM implemented the field study in rural Malawi. RM and MIO contributed towards laboratory analyses of serum metabolites. All authors contributed to the writing and approving the final manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgements
This work was supported by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital, the Hickey Family Foundation, the National Institutes of Health R01 AG27012 and the Intramural Branch of the National Institute on Aging, Baltimore, Maryland. The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
References
- Alp H., Orbak Z., Akçay F. Plasma and urine carnitine levels and carnitine supplementation in children with malnutrition. J. Trop. Pediatr. 1999;45:294–296. doi: 10.1093/tropej/45.5.294. [DOI] [PubMed] [Google Scholar]
- Bickler S.W., Ring J., De Maio A. Sulfur amino acid metabolism limits the growth of children living in environments of poor sanitation. Med. Hypotheses. 2011;77:380–382. doi: 10.1016/j.mehy.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Mach. Learn. 2001;45:5–32. [Google Scholar]
- Campbell D.I., Murch S.H., Elia M. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr. Res. 2003;54:306–311. doi: 10.1203/01.PDR.0000076666.16021.5E. [DOI] [PubMed] [Google Scholar]
- Carroll J.E., Brooke M.H., Shumate J.B. Carnitine intake and excretion in neuromuscular diseases. Am. J. Clin. Nutr. 1981;34:2693–2698. doi: 10.1093/ajcn/34.12.2693. [DOI] [PubMed] [Google Scholar]
- Chickos J.S., Way B.A., Wilson J. Analysis of 3-hydroxydodecanedioic acid for studies of fatty acid metabolic disorders: preparation of stable isotope standards. J. Clin. Lab. Anal. 2002;16:115–120. doi: 10.1002/jcla.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R.J., Jones K.D., Berkley J.A. Environmental enteric dysfunction: an overview. Food Nutr. Bull. 2015;36(1 Suppl):S76–S87. doi: 10.1177/15648265150361S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill C.M., Helms R.A. The use of carnitine in pediatric nutrition. Nutr. Clin. Pract. 2007;22:204–213. doi: 10.1177/0115426507022002204. [DOI] [PubMed] [Google Scholar]
- da Rosa M.S., Seminotti B., Ribeiro C.A. 3-Hydroxy-3-methylglutaric and 3-methylglutaric acids impair redox status and energy production and transfer in rat heart: relevance for the pathophysiology of cardiac dysfunction in 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency. Free Radic. Res. 2016;50:997–1010. doi: 10.1080/10715762.2016.1214952. [DOI] [PubMed] [Google Scholar]
- DeHaven C., Evans A., Dai H. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarquoy J., Georges B., Rigault C. Radioisotopic determination of l-carnitine content in foods commonly eaten in Western countries. Food Chem. 2004;2004(86):137–142. [Google Scholar]
- Denno D.M., VanBuskirk K., Nelson Z.C. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin. Infect. Dis. 2014;59(Suppl. 4):S213–S219. doi: 10.1093/cid/ciu541. [DOI] [PubMed] [Google Scholar]
- Dorland L., Ketting D., Bruinvis L. Medium- and long-chain 3-hydroxymonocarboxylic acids: analysis by gas chromatography combined with mass spectrometry. Biomed. Chromatogr. 1991;5:161–164. doi: 10.1002/bmc.1130050405. [DOI] [PubMed] [Google Scholar]
- Dror D.K., Allen L.H. The importance of milk and other animal-source foods for children in low-income countries. Food Nutr. Bull. 2011;32:227–243. doi: 10.1177/156482651103200307. [DOI] [PubMed] [Google Scholar]
- Evans A.M., Bridgewater B.R., Liu Q. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4:132. [Google Scholar]
- Evans A.M., DeHaven C.D., Barrett T. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Friedman J.H. Multivariate adaptive regression splines (with discussion) Ann. Stat. 1991;19:1–141. [Google Scholar]
- Friedman J.H. Greedy function approximation: a gradient boosting machine. Ann. Stat. 2001;29:1189–1232. [Google Scholar]
- Giesbertz P., Ecker J., Haag A. An LC-MS/MS method to quantify acylcarnitine species including isomeric and odd-numbered forms in plasma and tissues. J. Lipid Res. 2015;56:2029–2039. doi: 10.1194/jlr.D061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R.L., Leite A.M., Pinkerton R. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One. 2016;2016(11) doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B., West J.A., Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (c15:0) and heptadecanoic acid (c17:0) in health and disease. Molecules. 2015;20:2425–2444. doi: 10.3390/molecules20022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P., Besa E., Zyambo K. Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler B.S. Carnitine: an overview of its role in preventive medicine. Prev. Med. 1986;15:373–390. doi: 10.1016/0091-7435(86)90005-8. [DOI] [PubMed] [Google Scholar]
- Khan L., Bamji M.S. Tissue carnitine deficiency due to dietary lysine deficiency: triglyceride accumulation and concomitant impairment in fatty acid oxidation. J. Nutr. 1979;109:24–31. doi: 10.1093/jn/109.1.24. [DOI] [PubMed] [Google Scholar]
- Koeth R.A., Wang Z., Levison B.S. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M.N., Mduma E., Kosek P.S. Plasma tryptophan and the kynurenine-tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am.J.Trop. Med. Hyg. 2016;95:928–937. doi: 10.4269/ajtmh.16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs N.F., Miller L.V., Hambidge K.M. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatr. Int. Child Health. 2014;34:279–288. doi: 10.1179/2046905514Y.0000000151. [DOI] [PubMed] [Google Scholar]
- Lees H.J., Swann J.R., Wilson I.D. Hippurate: the natural history of a mammalian-microbial cometabolite. J. Proteome Res. 2013;12:1527–1546. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- Lerner A., Gruener N., Iancu T.C. Serum carnitine concentrations in coeliac disease. Gut. 1993;34:933–935. doi: 10.1136/gut.34.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord R.S., Bralley J.A., editors. Laboratory Evaluations for Integrative and Functional Medicine. Metametrix Institute; Duluth, Georgia: 2008. [Google Scholar]
- Mamedov I., Zolkina I., Nikolaeva E. Carnitine insufficiency in children with inborn errors of metabolism: prevalence and treatment efficacy. J. Pediatr. Endocrinol. Metab. 2015;28:1299–1304. doi: 10.1515/jpem-2015-0193. [DOI] [PubMed] [Google Scholar]
- Martin F.P., Ezri J., Cominetti O. Urinary metabolic phenotyping reveals differences in the metabolic status of healthy and inflammatory bowel disease (IBD) children in relation to growth and disease activity. Int. J. Mol. Sci. 2016;17(8) doi: 10.3390/ijms17081310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler P.E., Stoll M.S., Ingalls S.T. Validated method for the quantification of free and total carnitine, butyrobetaine, and acylcarnitines in biological samples. Anal. Chem. 2015;87:8994–9001. doi: 10.1021/acs.analchem.5b02198. [DOI] [PubMed] [Google Scholar]
- Miura Y. The biological significance of ω-oxidation of fatty acids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013;89:370–382. doi: 10.2183/pjab.89.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri M., Mottaghi Moghadam Shahri H., Horri M. Absolute and relative carnitine deficiency in patients on hemodialysis and peritoneal dialysis. Iran. J. Kidney Dis. 2016;10:36–43. [PubMed] [Google Scholar]
- Nuss E.T., Tanumihardjo S.A. Quality protein maize for Africa: closing the protein inadequacy gap in vulnerable populations. Adv. Nutr. 2011;2:217–224. doi: 10.3945/an.110.000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A.L., Nelson S.E., Rebouche C.J. Low carnitine intake and altered lipid metabolism in infants. Am. J. Clin. Nutr. 1989;49:624–628. doi: 10.1093/ajcn/49.4.624. [DOI] [PubMed] [Google Scholar]
- Onkenhout W., Venizelos V., van der Poel P.F. Identification and quantification of intermediates of unsaturated fatty acid metabolism in plasma of patients with fatty acid oxidation disorders. Clin. Chem. 1995;41:1467–1474. [PubMed] [Google Scholar]
- Ordiz M.I., Stephenson K., Agapova S. Environmental enteric dysfunction and the fecal microbiota in Malawian children. Am.J.Trop. Med. Hyg. 2016 doi: 10.4269/ajtmh.16-0617. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpão R.C., Ventura M.R., Ferreira R.B. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015;113:454–463. doi: 10.1017/S0007114514003511. [DOI] [PubMed] [Google Scholar]
- Poesen R., Claes K., Evenepoel P. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J. Am. Soc. Nephrol. 2016;27:3479–3487. doi: 10.1681/ASN.2015121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisac B., Arnedo M., Casale C.H. Differential HMG-CoA lyase expression in human tissues provides clues about 3-hydroxy-3-methylglutaric aciduria. J. Inherit. Metab. Dis. 2010;33:405–410. doi: 10.1007/s10545-010-9097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachakonda V., Gabbert C., Raina A. Serum metabolomic profiling in acute alcoholic hepatitis identifies multiple dysregulated pathways. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S.E., Evans A.M. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012;51:553–572. doi: 10.1007/BF03261931. [DOI] [PubMed] [Google Scholar]
- Roberts L.D., Boström P., O'Sullivan J.F. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W.R., Duncan S.H., Scobbie L. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol. Nutr. Food Res. 2013;57:523–535. doi: 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- Seakins J.W. The determination of urinary phenylacetylglutamine as phenylacetic acid. Studies on its origin in normal subjects and children with cystic fibrosis. Clin. Chim. Acta. 1971;35:121–131. doi: 10.1016/0009-8981(71)90302-0. [DOI] [PubMed] [Google Scholar]
- Semba R.D., Shardell M., Sakr Ashour F.A. Child stunting is associated with low circulating essential amino acids. EBioMedicine. 2016;6:246–252. doi: 10.1016/j.ebiom.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba R.D., Shardell M., Trehan I. Metabolic alterations in children with environmental enteric dysfunction. Sci. Rep. 2016;6:28009. doi: 10.1038/srep28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba R.D., Zhang P., Gonzalez-Freire M. The association of serum choline with linear growth failure in young children from rural Malawi. Am. J. Clin. Nutr. 2016;104:191–197. doi: 10.3945/ajcn.115.129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambat K., Moudgil A. Carnitine deficiency in children receiving continuous renal replacement therapy. Hemodial. Int. 2016;20:63–67. doi: 10.1111/hdi.12341. [DOI] [PubMed] [Google Scholar]
- Shekhawat P.S., Sonne S., Carter A.L. Enzymes involved in l-carnitine biosynthesis are expressed by small intestinal enterocytes in mice: implications for gut health. J. Crohns Colitis. 2013;7:e197–e205. doi: 10.1016/j.crohns.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat P.S., Srinivas S.R., Matern D. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(−/−)) mice. Mol. Genet. Metab. 2007;92:315–324. doi: 10.1016/j.ymgme.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalet D.L., Martin G.R., Poole A. Differential sugar absorption as a marker for adaptation in short bowel syndrome. J. Pediatr. Surg. 2000;35:661–664. doi: 10.1053/jpsu.2000.5937. [DOI] [PubMed] [Google Scholar]
- Sonne S., Shekhawat P.S., Matern D. Carnitine deficiency in OCTN2 −/− newborn mice leads to a severe gut and immune phenotype with widespread atrophy, apoptosis and a pro-inflammatory response. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley C.A. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 2004;1033:42–51. doi: 10.1196/annals.1320.004. [DOI] [PubMed] [Google Scholar]
- Storey J.D. A direct approach to false discovery rates. J. R. Stat. Soc. Ser. B. 2002;64:479–498. [Google Scholar]
- Storey J.D., Taylor J.E., Siegmund D. Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J. R. Stat. Soc. Ser. B. 2004;66:187–205. [Google Scholar]
- Tanner L.M., Näntö-Salonen K., Rashed M.S. Carnitine deficiency and l-carnitine supplementation in lysinuric protein intolerance. Metabolism. 2008;57:549–554. doi: 10.1016/j.metabol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tanphaichitr V., Broquist H.P. Lysine deficiency in the rat: concomitant impairment in carnitine biosynthesis. J. Nutr. 1973;103:80–87. doi: 10.1093/jn/103.1.80. [DOI] [PubMed] [Google Scholar]
- Tein I. Disorders of fatty acid oxidation. Handb. Clin. Neurol. 2013;113:1675–1688. doi: 10.1016/B978-0-444-59565-2.00035-6. [DOI] [PubMed] [Google Scholar]
- Trehan I., Kelly P., Shaikh N. New insights into environmental enteric dysfunction. Arch. Dis. Child. 2016;101:741–744. doi: 10.1136/archdischild-2015-309534. [DOI] [PubMed] [Google Scholar]
- van der Laan M.J., Polley E.C., Hubbard A.E. Super learner. Stat. Appl. Genet. Mol. Biol. 2007;6 doi: 10.2202/1544-6115.1309. (Article 25) [DOI] [PubMed] [Google Scholar]
- Watanabe K., Petri W.A., Jr. Environmental enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine. 2016;10:25–32. doi: 10.1016/j.ebiom.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz A.J., Manary M.J., Stephenson K. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J. Pediatr. Gastroenterol. Nutr. 2012;55:747–750. doi: 10.1097/MPG.0b013e3182650a4d. [DOI] [PubMed] [Google Scholar]
- Wierzbicki A.S., Mayne P.D., Lloyd M.D. Metabolism of phytanic acid and 3-methyl-adipic acid excretion in patients with adult Refsum disease. J. Lipid Res. 2003;44:1481–1488. doi: 10.1194/jlr.M300121-JLR200. [DOI] [PubMed] [Google Scholar]
- Winter S.C., Szabo-Aczel S., Curry C.J. Plasma carnitine deficiency. Clinical observations in 51 pediatric patients. Am. J. Dis. Child. 1987;141:660–665. doi: 10.1001/archpedi.1987.04460060076039. [DOI] [PubMed] [Google Scholar]
- Yu J., Ordiz M.I., Stauber J. Environmental enteric dysfunction includes a broad spectrum of inflammatory responses and epithelial repair processes. Cell. Mol. Gastroenterol. Hepatol. 2015;2:158–174.e1. doi: 10.1016/j.jcmgh.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Classification modeling using Super Learner algorithm
Spearman correlations of serum metabolites with L:M ratio
Serum metabolites in children with and without EED