Figure 3. Early administration of rNRG1 reduces myocardial death and stimulates cardiomyocyte proliferation.
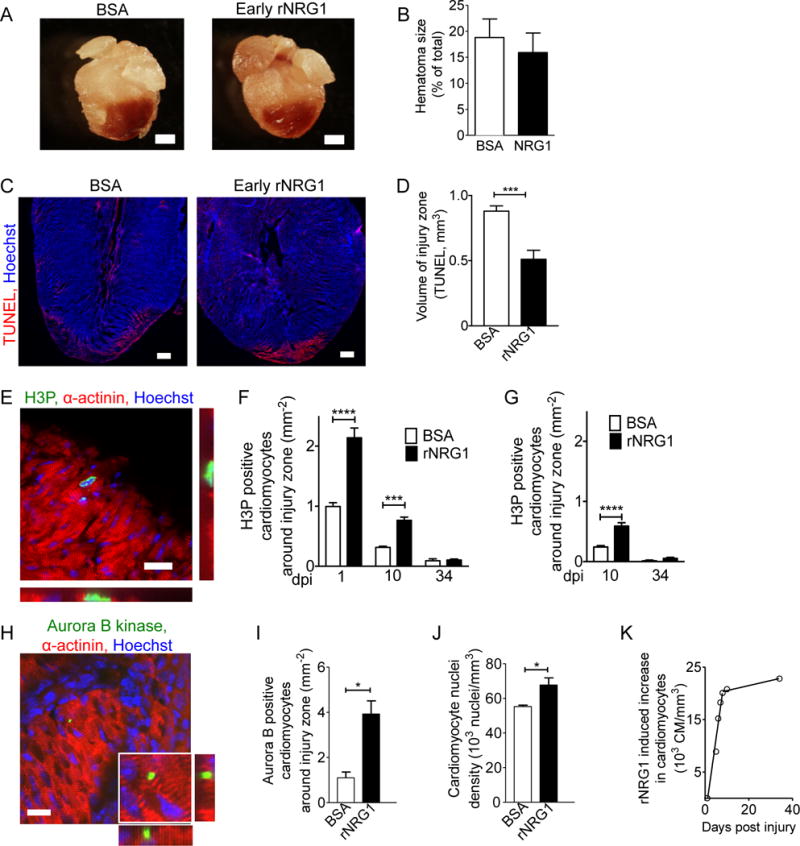
(A,B) Hematomas are present at the zone of injury at 1 dpi (A). Hematoma size quantification shows no change after early administration of rNRG1 (B). (C,D) Photomicrographs (C) and quantification (D) of myocardial cell death visualized by TUNEL staining at 1 dpi after early administration. (E) Cardiomyocytes in M-phase were visualized with an antibody against phosphorylated histone H3 (H3P). (F,G) H3P-positive cardiomyocytes were quantified around injury zone after early (F) and late (G) administration. Treatment with rNRG1 increases cardiomyocyte cell cycle activity, and early administration captures the regenerative phase (F). (H,I) Cardiomyoctyes in cytokinesis were visualized with an antibody against Aurora B kinase (H) and quantified around the injury zone after early administration at 1 dpi (I). (J) Cardiomyocyte nuclear density is increased after early administration of rNRG1 (34 dpi). (K) Early administration of rNRG1 increases the cardiomyocyte density by ~62,000 cardiomyocytes/mm3 within the first 8 days, compared to BSA controls. Scale bars 1 mm (A), 20 μm (C,E,H). Statistical significance was tested with t-test (B,D,I,J) and ANOVA (F,G) * P < 0.05, *** P < 0.001, **** P < 0.0001.
