Abstract
The frequency and the geographical extent of symbiotic associations between ants and fungi of the order Chaetothyriales have been highlighted only recently. Using a phylogenetic approach based on seven molecular markers, we showed that ant-associated Chaetothyriales are scattered through the phylogeny of this order. There was no clustering according to geographical origin or to the taxonomy of the ant host. However, strains tended to be clustered according to the type of association with ants: strains from ant-made carton and strains from plant cavities occupied by ants (‘domatia’) rarely clustered together. Defining molecular operational taxonomic units (MOTUs) with an internal transcribed spacer sequence similarity cut-off of 99% revealed that a single MOTU could be composed of strains collected from various ant species and from several continents. Some ant-associated MOTUs also contained strains isolated from habitats other than ant-associated structures. Altogether, our results suggest that the degree of specialization of the interactions between ants and their fungal partners is highly variable. A better knowledge of the ecology of these interactions and a more comprehensive sampling of the fungal order are needed to elucidate the evolutionary history of mutualistic symbioses between ants and Chaetothyriales.
Keywords: symbiosis, mutualism, ant-plant, Chaetothyriales, phylogeny
1. Introduction
Fungi of the order Chaetothyriales (Ascomycetes), also referred to as ‘black yeasts and relatives’, are mostly known from a range of oligotrophic or extreme environments, such as bare rocks, plant surfaces, indoor surfaces of buildings and substrates contaminated with aromatic hydrocarbons [1–4]. Some Chaetothyriales are also opportunistic human pathogens causing various diseases ranging from skin to neurotropic infections [5]. Their extremophilic adaptation—high resistance to a physical or chemical stress—is accompanied by very low competitive ability in mild, buffered environments. Several strains of Chaetothyriales have recently been found in symbiotic association with ants, but these remain poorly investigated. Most of them appear to be species new to science. As ants produce a large diversity of secondary metabolites, it has been suggested that Chaetothyriales might be predisposed to occupy ant nests because of their particular capacity to metabolize various chemical substances, including aromatic hydrocarbons and other secondary metabolites [6,7]. As ants are expected to shape their microbial environment by the production of multiple anti-microbial substances, investigating these associations will contribute to elucidating the processes underlying the evolution of insect–microbe symbioses, for which convergence is a pervasive theme in insect diversification [8,9].
Ant-associated Chaetothyriales can be classified into three main functional groups: (i) strains associated with fungus-growing attine ants [10–12]; (ii) strains involved in ‘carton’ structures built by ants [7,13–15]; and (iii) strains associated with ant–plant symbioses [7,16]. The first group, Chaetothyriales associated with attine ants, are not the focus of our study. However, because our analysis allows a comparison of their phylogenetic placement, they are briefly presented here. The nature of the association between Chaetothyriales and attine ants is mostly unknown. It was first proposed that Chaetothyriales were detrimental to the symbiosis between attine ants and their cultivated fungi because they were shown to lower the growth of a specific bacterium (Pseudonocardia) known to protect the cultivated fungus through the production of antibiotics [17]. However, the proposition that black yeasts inhibit Pseudonocardia was based on in vitro experiments confronting these two organisms on artificial medium. Since then, the hypothesis of coevolution between attine ants and Pseudonocardia has been challenged and the system is now viewed as a complex community of bacteria recruited by the ants from the environment [18]. The role of Chaetothyriales in this context has not yet been re-investigated. Nevertheless, evidence of the presence of Chaetothyriales on the cuticle of attine ants is accumulating [11,12].
Several ant species make a cardboard-like material (‘carton’) to build their entire nest, partition the space available in natural cavities, cover and protect their hemipteran trophobionts, or construct runways and galleries for protection or for capturing large prey. Fungal mycelium growing within ant carton—and cultured by the ants themselves—has long been known to contribute to structural support, stabilizing the construction material used as carton components (i.e. plant debris or fibres) [19–23]. However, the taxonomic affiliation of these fungi was unknown until recently and the frequency and extent of associations with ants were underestimated. Fungi of various orders (e.g. Botryosphaeriales, Eurotiales, Capnodiales, Chaetothyriales, Hypocreales, Pleosporales) can be isolated from carton. Only strains of the orders Chaetothyriales and Capnodiales are isolated from pieces of hyphae and are thus considered to have a structural function in carton construction [7,13–15]. The other strains have been isolated only from spores and are most likely contaminants. Numerous strains of Chaetothyriales are usually mixed within a single piece of carton, and show low specificity in regard to the identity of the carton-making ant [15,23]. Carton-making is widespread among tropical ants, but also occurs in temperate species [7,14,19]. Some ground-dwelling ants construct carton, but carton-making is most widespread in arboreal ants, with some ant genera being particularly renowned for building carton structures (e.g. Crematogaster, Azteca, Camponotus, Polyrhachis). The capacity to build carton is scattered throughout the ant phylogeny. It has either evolved and/or been lost several times independently, or is facultative or sporadic for all ants.
Chaetothyriales are astonishingly abundant in tropical ant–plant symbioses. In these symbioses, the plants host ants in specialized hollow structures called domatia. Such symbioses are restricted to the tropics, but widespread throughout tropical regions. The ant colony usually lives within the host plant throughout its whole lifespan. Domatia have various anatomical origins that vary across taxa; these include twigs, leaves and stipules [24]. In addition to providing ants with nesting sites, ant-plants also often contribute to ant nutrition, either directly through the production of extrafloral nectar and food bodies, or indirectly through sap-sucking Hemiptera that ants rear in domatia for honeydew and ‘meat’ [25]. Ant-plants benefit from the presence of ants in three ways: (i) protection against herbivores (vertebrates and/or invertebrates) and pathogens, (ii) reduced competition through pruning of the surrounding vegetation and (iii) uptake of nitrogen and possibly other nutrients from ant activity [16,24,26]. While the occurrence of fungi within ant-occupied domatia has long been recorded, the nature of these fungi and of their relationship with ant–plant symbioses has only recently been investigated [16,27]. The inner wall of each domatium has a dark, thin patch where the fungal symbionts are located. Fungal patches within domatia are mainly constituted of a few strains of Chaetothyriales that initially appeared to be more or less specific to the ant species [7,27–29]. Fungal patches also contain nematodes, bacteria and spores of opportunistic fungi [30–32]. Detailed functional investigation of a few systems revealed that domatia patches are manured and used as a food source by the ants [33,34] in a way reminiscent of fungal culture by attine ants. Ants and plants living in symbiosis are scattered throughout their respective phylogenies and have evolved many times independently, at least 40 and 158 times in their respective lineages [24,35]. Ant–plant symbioses evolved recently (probably not before the Miocene) and are rarely associated with substantial speciation of plant or ant lineages [35,36].
A previous phylogenetic study indicated that ant-associated Chaetothyriales form a few monophyletic clades, and that each domatia strain was associated with only one ant species, leading the authors to postulate that ant-associated Chaetothyriales from domatia were ant-species-specific and specialized to this lifestyle [7]. The rapid rate at which ant-associated species of Chaetothyriales are being discovered suggests that ant-Chaetothyriales associations are extremely frequent. A reappraisal of the phylogenetic distribution of ant-associated strains is needed because apparent ant-specificity could result from the under-sampling of this poorly known order of fungi. Here, we reconstruct a new multi-gene phylogeny of the Chaetothyriales based on one mitochondrial and three nuclear genes. In addition, we reconstruct phylogenies of subsets of strains using three additional, more variable nuclear markers. Our phylogenetic approach includes ant-associated strains from ant–plant associations that had not been analysed in previous studies (about one-third of the strains are new). This study also includes a larger number of Chaetothyriales strains not associated with ants, improving the ability of our phylogeny to clarify the phylogenetic position of ant-associated strains.
2. Methods
(a). Sampling
Our dataset included a total of 242 strains of Chaetothyriales (electronic supplementary material, table S1): 46 strains from ant-made carton (among which seven are new isolates), 55 strains from domatia patches (among which 25 are new isolates) and 141 strains of various origins intended to cover the broadest possible range of functional and taxonomic diversity in the order Chaetothyriales. Our choice of strains not associated with ants was constrained by the availability of already published sequences. We cannot exclude the possibility that additional strains would change the topology of the phylogenetic trees of this study. As outgroups, we used five taxa in the order Verrucariales according to the phylogeny of Gueidan et al. [37].
Seven gene regions were used as phylogenetic markers: the small and the large subunits of the nuclear ribosomal RNA gene (nSSU and nLSU, respectively), the small subunit of the mitochondrial ribosomal RNA gene (mtSSU), the fragments AC and DF of the largest subunit of the RNA polymerase II (RPB1), the internal transcribed spacer region (ITS) composed of ITS1, 5.8S and ITS2, the partial beta tubulin gene (Bt2) and the translation elongation factor 1 alpha (EF1). Sequences were produced de novo using the protocols described below or retrieved from GenBank. Many strains with missing information were included in the phylogenetic analyses, in particular strains not associated with ants. We decided to include these strains because of their potential importance for our study according to their taxonomic position or ecology. For more details on missing data, see the electronic supplementary material, table S1 and alignment matrices at TreeBase. Collection of samples of ant-made carton and domatia fungal patches, isolation of fungal strains and DNA extraction from pure cultures followed the protocols described in Voglmayr et al. [7]. Strains and GenBank accession numbers used in this study are listed in the electronic supplementary material, table S1.
(b). DNA amplification and sequencing
Amplification by polymerase chain reaction (PCR) was performed using either the Qiagen multiplex kit (Qiagen, Venlo, Netherlands) or the Sigma REDExtract-N-Amp PCR Ready Mix (Sigma-Aldrich). Conditions for PCR first followed manufacturer's instructions, and were then adjusted if amplification failed. Amplification used 25 µl of solution containing 12.5 µl of PCR mix (from either the Qiagen or Sigma kits), 5 µl of Q solution or water (Qiagen and Sigma respectively), 2.5 µl of each primer at 5 µM, and 2.5 µl of DNA extract. Primers used for amplification and sequencing are in the electronic supplementary material, table S2. The PCR programme was: 15 min at 95°C, 35 cycles of 1 min at 94°C, 1 min at 45–55°C, 1.5–3 min at 72°C (depending on expected sequence length), and a final elongation of 20 min at 60°C. Sanger dideoxy sequencing of the PCR products was performed at the Genoscope (Evry, France). Sequences were edited using CodonCode Aligner (CodonCode Corporation, Dedham, MA, USA), and contigs were built from forward and reverse sequences generated for each gene. Conflicting base calls were coded using the ambiguity code.
(c). Phylogenetic analyses
The sequences were aligned manually using Mesquite v. 3.04 [38]. We delimited and excluded from the alignments introns and all ambiguously aligned regions following the protocol of Lutzoni et al. [39].
We first used the four least variable markers (nSSU, nLSU, mtSSU, RPB1 region A-C and region D-F) to build the order-level phylogeny with all strains. To test for congruence between these markers, we ran maximum-likelihood (ML) analyses on each marker matrix with RAxML HPC2 v. 8.2.4 [40] on the Cipres Web Portal [41] using a GTR + I + G model and 1000 bootstrap pseudo-replicates. RPB1 regions were partitioned according to the three positions of the codons. Bootstrap consensus trees of each marker were compared by eye for sets of taxa supported by bootstrap values equal to or above 70%. We excluded the mtSSU sequences of strains CTeY1 and CTeY9 owing to incongruent placement. We then combined the four markers into a concatenated matrix and ran a ML analysis to investigate the phylogenetic relationships among the taxa. This ML analysis was conducted on the Cipres Web Portal with RAxML HPC2 v. 8.2.4 using a GTR + I + G model on six partitions (nSSU, nLSU, mtSSU, first, second and third codon positions of RPB1) as estimated by the Akaike Information Criterion in jModelTest2 v. 2.1.9 [42,43], and for 1000 bootstrap pseudo-replicates. In addition, we ran a Bayesian analysis on the concatenated matrix with MrBayes v. 3.2.6 [44] using the same model and partitions as in the ML analysis. Two analyses of four chains were run for 10 000 000 generations, sampling trees every 500 generations with 25% burn-in samples discarded for each run.
Then, to better resolve the phylogenetic relationships among closely related strains, we built phylogenies of subsets of taxa based on three markers, ITS, Bt2 and EF1, containing parts that are more variable than the markers used for the order-level phylogeny. In each subset, we included additional strains that were not in the order-level phylogeny, and for which we retrieved sequences from GenBank. We did not remove introns and indels in the alignments of these markers. For each subset, we ran ML analysis on each matrix as described above. After checking the congruence between the markers, we concatenated the matrices and ran ML analysis. For consistency, we used the same parameters and models as for the main tree. The ITS region is considered to be the most relevant DNA barcode for fungi [45]. In order to detect Chaetothyriales species that could potentially occur in association with several ant species, and/or on several continents, we delineated molecular operational taxonomic units (MOTUs) in ant-associated strains using a cut-off similarity of 99% between ITS sequences. As our concern was to group strains that were highly likely to belong to a single species, we chose a cut-off value in the upper range observed for Eurotiomycetes [45]. A lower value increases the risk of false conclusion regarding the range of potential hosts and the extent of geographical distribution of a particular species. The distribution curve of pairwise ITS similarity for the 38 ant-associated strains in the family Cyphellophoraceae (which proved to form a monophyletic clade, see below) shows a drop around the 99% value, but not around the 97% value, indicating that the traditional cut-off value of 97% is less consistent with discontinuity in sequence similarity than the 99% value used in our study (electronic supplementary material, figure S1). The drop of the distribution curve is interpreted as the transition between the average intraspecific and the average interspecific sequence similarity.
3. Results
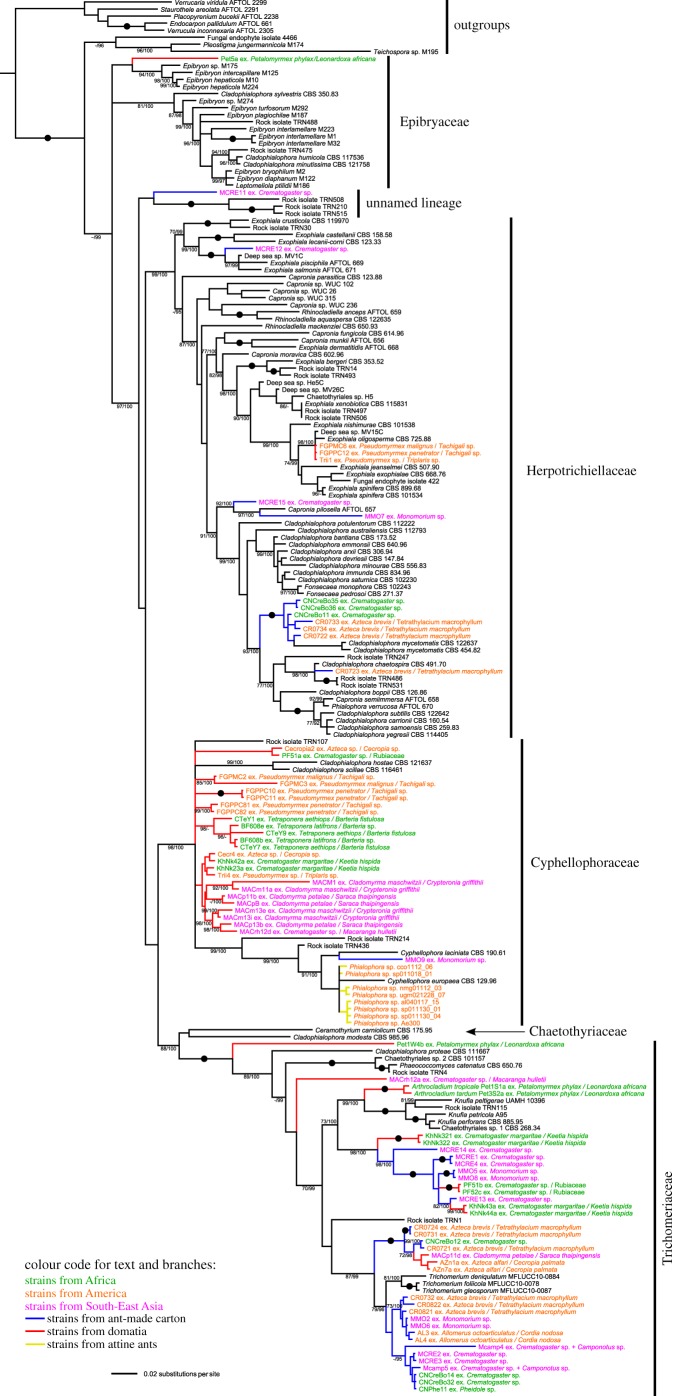
For this study, we generated 93 mtSSU, 27 RPB1, 88 nSSU, 81 nLSU, 79 ITS, 61 EF1 and 58 Bt2 new sequences. GenBank accessions of new sequences are given in the section on data accessibility and in the electronic supplementary material, table S1. The dataset of the order-level phylogeny based on four markers included 6292 characters (1290 for nLSU, 1580 for nSSU, 596 for mtSSU and 2826 for RBP1). Bayesian and ML analyses yielded similar trees: we present only the tree from the Bayesian analysis (figure 1). The accession URL of the TreeBase project with alignments and phylogenetic trees is indicated in the section on data accessibility. The families Trichomeriaceae, Herpotrichiellaceae and Cyphellophoraceae, in which most ant-associated strains are located, formed well-supported clades.
Figure 1.
Phylogenetic reconstruction of the Chaetothyriales based on a Bayesian approach and four markers (nLSU, nSSU, mtSSU, RPB1), showing the position of strains isolated from ant nests (ant-made carton, ant-occupied domatia of plants symbiotically associated with ants, or attine ants). Strong support values (100% bootstrap (BS) and posterior probabilities (PP)) are indicated by black dots. Other values are indicated as BS/PP. Dashes replace non-significant values (less than 70% for BS and less than 95% for PP). Each label of an ant-associated strain corresponding to an undescribed species from carton and domatia is composed of the name of the strain, the name of the ant and the name of the host plant (if any). Names of strains from Africa, America and Southeast Asia are represented in green, orange and magenta, respectively. Strains from carton, domatia and attine ants have blue, red and yellow branches, respectively.
Ant-associated strains were scattered throughout the phylogeny and branched at various depths in the phylogeny (figure 1). Most strains clustered according to whether they originated from carton or from domatia, although a few clades in Trichomeriaceae included both functional types. Ant-associated strains occurred in four out of the five formally described families of Chaetothyriales, although most of them belonged to the Trichomeriaceae, followed by the Cyphellophoraceae, and to a lesser extent by the Herpotrichiellaceae. Various types of association with ants were found in each of these three families. Strains from both domatia patches and ant-made carton occurred in Trichomeriaceae and Herpotrichiellaceae. The Cyphellophoraceae contained most of the strains from domatia, but also strains isolated from the cuticle of attine ant workers and gynes in previous studies [10,11]. Although there were several independent origins of each functional type, most of the ant-associated strains appeared to cluster in a few clades. For instance, the Trichomeriaceae contained a monophyletic clade of carton strains (figure 1) and a large clade with strong support in the Cyphellophoraceae was composed only of domatia strains (figure 2a).
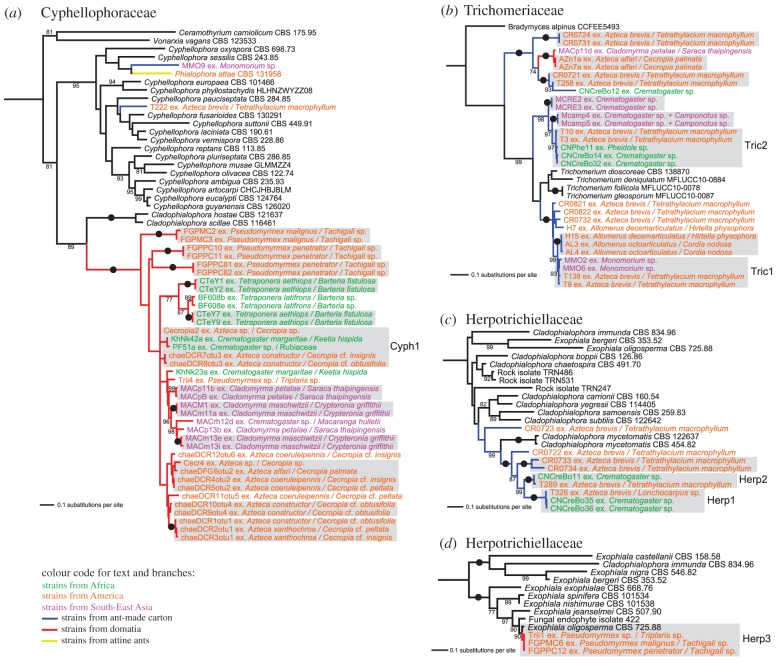
Figure 2.
Phylogenetic trees of subsets of Chaetothyriales strains generated by maximum-likelihood analysis based on three markers (ITS, Bt2, EF1). (a) Subset for the family Cyphellophoraceae. (b) Subset for the family Trichomeriaceae. (c) and (d) Subsets for the family Herpotrichiellaceae. Clades corresponding to MOTUs (more than 99% ITS similarity) are highlighted in grey. Those MOTUs that contain strains from various ant genera or continents are named. Bootstrap values above 70% are added to supported branches. Strong support values (100% BS) are indicated by black dots. Each label of an ant-associated strain corresponding to an undescribed species from carton or domatia is composed of the name of the strain, the name of the ant and the name of the host plant (if any). Names of strains from Africa, America and Southeast Asia are represented in green, orange and magenta, respectively. Strains from carton, domatia and attine ants have blue, red and yellow branches, respectively.
Our order-level phylogeny clearly showed the absence of phylogenetic clustering of the ant-associated strains according to their geographical origin (figure 1). As illustrated in several clades, strains from two or three continents were sometimes closely related. Similarly, there was no clustering of strains according to the taxonomic relationships between host ants or between host plants. A highly illustrative example is a monophyletic clade in the Trichomeriaceae composed of closely related strains from Central American Azteca (Dolichoderinae) plant-ants on Cecropia (Urticaceae) and Tetrathylacium (Salicaceae) (domatia and carton fungi), southeast Asian Cladomyrma (Formicinae) plant-ants on Saraca (Fabaceae) (domatia fungi) and African Crematogaster (Myrmicinae) (carton fungi) (figure 1).
Phylogenies based on subsets of strains (figure 2) and computation of ITS sequence similarity revealed seven cases—two of which had already been noted by Nepel et al. [15]—of a single MOTU (more than 99% ITS sequence similarity) comprised of strains isolated from several ant species and/or on several continents. One MOTU (strain T394 from Nepel et al. [15] and MCRE12) was found in carton nests of Crematogaster sp. in Thailand and in carton galleries of Azteca brevis in Costa Rica (for illustration see Nepel et al. [15]). Two MOTUs from Azteca nests in Costa Rica, Cyph1 (figure 2a) and Tric1 (figure 2b), were found respectively in domatia of Keetia hispida and a closely related Rubiaceae occupied by Crematogaster margaritae in Cameroon, and in carton nests of Monomorium sp. in Malaysia. Three MOTUs (Tric2, Herp1 and Herp2; figure 2b,c) were found in carton nests of Crematogaster sp. and/or Pheidole sp. in Cameroon and in carton galleries of Azteca brevis in Costa Rica. Finally, one MOTU (Herp3, figure 2d), which corresponds to the species Exophiala oligosperma, was found in domatia of Tachigali sp. (Fabaceae) occupied by Pseudomyrmex malignus or P. penetrator in French Guiana, and in domatia of Triplaris sp. (Polygonaceae) occupied by Pseudomyrmex sp. in Costa Rica.
4. Discussion
Our multilocus phylogeny of the Chaetothyriales based on a sample of taxa representative of the entire diversity of the order shows a pattern of phylogenetic scatter of ant-associated strains. These strains do not cluster according to their geographical origin nor to the identity of their species of host ant or host plant. Ant-associated fungal taxa are not necessarily host-specific, but can occur in niches other than ant nests, and can be distributed worldwide. Many aspects of the evolutionary history of ant-associated Chaetothyriales are still unclear. More data on the nature of the interaction between ants and particular Chaetothyriales taxa (for example, distinguishing mutualistic interactions from others), and more comprehensive sampling (for example, to infer ancestral habitats of ant species-specific taxa) will be required to resolve open questions.
Studies on the pattern of association between ants and Chaetothyriales at both local and larger spatial scales can contribute greatly to understanding the ecology of ant-associated strains. In Cameroon, we investigated Chaetothyriales associated with three ant-plant symbioses co-occurring at the same site: Petalomyrmex phylax on Leonardoxa africana subsp. africana, Tetraponera aethiops on Barteria fistulosa and Crematogaster margaritae on Keetia hispida. We found that each ant–plant symbiosis had its own set of domatia fungal strains [7,28]. In Central America, four Azteca species living with three different Cecropia species were investigated along a transect of a few kilometres length. Although some strains were shared across Azteca species, some others were ant-species-specific [27]. By contrast, the present study shows that several MOTUs are composed of strains isolated from various ant species in various continents, suggesting both low ant-specificity and long-distance dispersal of ant-associated Chaetothyriales. Moreover, strains found in domatia of Tachigali (in French Guiana) and Triplaris (in Costa Rica) occupied by different Pseudomyrmex ant species both correspond to Exophiala oligosperma (99.4% ITS sequence similarity), a fungus isolated from various low-nutrient environmental substrates, reported to be an opportunistic human pathogen and known to metabolize toluene [46,47]. In addition, a recently described species, Arthrocladium tardum (Trichomeriaceae), has been isolated both from a domatium of Leonardoxa africana occupied by the ant Petalomyrmex phylax in Cameroon and from decaying coconut shells in Brazil [6]. Although some Chaetothyriales strains are clearly mutualistic symbionts of ants [28,33,34], our study indicates that ant nests can be colonized by opportunistic species and that Chaetothyriales taxa might display exceptional dispersal capacities and surprising ubiquity. In the future, it will be most useful to investigate the degree of specialization of the interactions between particular ant and fungal partners in order to interpret the phylogenetic pattern in evolutionary terms.
The order-level phylogeny shows that the strains we isolated from ant-made carton or from domatia occupied by plant-associated ants are scattered throughout the phylogeny of the order Chaetothyriales. Among the ant-associated strains added by this study to the phylogeny published by Voglmayr et al. [7], two (MACrh12a and Pet5a) form long and isolated branches occurring basally in their respective families (Trichomeriaceae and Epibryaceae). Epiphyllous strains recently described in the genus Trichomerium (Trichomeriaceae) [4] are placed within a clade that previously was known to contain only strains from ant-made carton [7]. In addition to a better knowledge of the functional ecology of ant-Chaetothyriales interactions, we need a more comprehensive sampling of strains in the phylogeny to properly infer the evolutionary history of cases of mutualistic symbioses between ants and Chaetothyriales. Our results suggest that focusing on communities of epiphylls should be the next step to elucidate the functional ecology of black yeasts and their roles in the biology of ants.
5. Conclusion
The molecular characterization of fungi associated with tropical arboreal ants opens a window on an unsuspected aspect of the evolution and ecology of the Chaetothyriales. In addition, it reveals complex patterns of association with ants. The functional and evolutionary ecology underlying these patterns remains to be understood. Accomplishing this will require screening the environments surrounding nests of tropical arboreal ants—especially the plant hosts of these ants—for Chaetothyriales, and investigating the mode of transmission of the fungal symbiont. Preliminary results suggest that founding queens may carry fungi from their mother colony in their infrabuccal pockets [48]. Tropical arboreal ants aside, Chaetothyriales have also been found on the cuticles of attine ants and in carton structures of ground-dwelling ants of temperate latitudes. Are these fungi universal symbionts of ants? We may have just barely seen the tip of the iceberg.
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
DNA sequences: GenBank accessions KX822178-KX822578. Phylogenetic data, including alignments: TreeBase accession URL http://purl.org/phylo/treebase/phylows/study/TB2:S19475.
Authors' contributions
R.B., V.M., H.V. and M.N. carried out field collection and isolation of fungal strains; M.V., R.B., H.V., M.N., V.M. and L.M. carried out the molecular laboratory work; M.V. and C.G. carried out sequence alignments; M.V., R.B. and C.G. participated in data analysis; R.B., V.M., M.-A.S., D.M., C.G. and S.d.H. conceived and designed the study; M.V. and R.B. drafted the manuscript. All authors improved the manuscript and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This project was supported by the network ‘Bibliothèque du Vivant’ funded by the CNRS, the Muséum National d'Histoire Naturelle, the INRA and the CEA (Centre National de Séquençage).
References
- 1.Gueidan C, Aptroot A, da Silva Caceres ME, Badali H, Stenroos S. 2014. A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes, Ascomycota). Mycol. Prog. 13, 1027–1029. ( 10.1007/s11557-014-0990-2) [DOI] [Google Scholar]
- 2.Prenafeta-Boldú FX, Summerbell R, de Hoog GS. 2006. Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiol. Rev. 30, 109–130. ( 10.1111/j.1574-6976.2005.00007.x) [DOI] [PubMed] [Google Scholar]
- 3.Badali H, Prenafeta-Boldu FX, Guarro J, Klaassen CH, Meis JF, De Hoog GS. 2011. Cladophialophora psammophila, a novel species of Chaetothyriales with a potential use in the bioremediation of volatile aromatic hydrocarbons. Fungal Biol. 115, 1019–1029. ( 10.1016/j.funbio.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 4.Chomnunti P, Ko TWK, Chukeatirote E, Hyde KD, Cai L, Gareth Jones EB, Kodsueb R, Hassan BA, Chen H. 2012. Phylogeny of Chaetothyriaceae in northern Thailand including three new species. Mycologia 104, 382–395. ( 10.3852/11-066) [DOI] [PubMed] [Google Scholar]
- 5.de Hoog GS, et al. 2000. Black fungi: clinical and pathogenic approaches. Med. Mycol. 38, 243–250. ( 10.1080/mmy.38.s1.243.250) [DOI] [PubMed] [Google Scholar]
- 6.Nascimento MMF, et al. 2016. Arthrocladium, an unexpected human opportunist in Trichomeriaceae (Chaetothyriales). Fungal Biol. 120, 207–218. ( 10.1016/j.funbio.2015.08.018) [DOI] [PubMed] [Google Scholar]
- 7.Voglmayr H, Mayer V, Maschwitz U, Moog J, Djiéto-Lordon C, Blatrix R. 2011. The diversity of ant-associated black yeasts: insights into a newly discovered world of symbiotic interactions. Fungal Biol. 115, 1077–1091. ( 10.1016/j.funbio.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 8.Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. 2005. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595. ( 10.1146/annurev.ecolsys.36.102003.152626) [DOI] [Google Scholar]
- 9.Gibson CM, Hunter MS. 2010. Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol. Lett. 13, 223–234. ( 10.1111/j.1461-0248.2009.01416.x) [DOI] [PubMed] [Google Scholar]
- 10.Little AEF, Currie CR. 2007. Symbiotic complexity: discovery of a fifth symbiont in the attine ant-microbe symbiosis. Biol. Lett. 3, 501–504. ( 10.1098/rsbl.2007.0253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attili-Angelis D, Duarte APM, Pagnocca FC, Nagamoto NS, de Vries M, Stielow JB, de Hoog GS. 2014. Novel Phialophora species from leaf-cutting ants (tribe Attini). Fungal Divers. 65, 65–75. ( 10.1007/s13225-013-0275-0) [DOI] [Google Scholar]
- 12.Duarte APM, Attili-Angelis D, Baron NC, Forti LC, Pagnocca FC. 2014. Leaf-cutting ants: an unexpected microenvironment holding human opportunistic black fungi. Antonie Van Leeuwenhoek 106, 465–473. ( 10.1007/s10482-014-0215-3) [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-González MX, Male PJG, Leroy C, Dejean A, Gryta H, Jargeat P, Quilichini A, Orivel J. 2011. Specific, non-nutritional association between an ascomycete fungus and Allomerus plant-ants. Biol. Lett. 7, 475–479. ( 10.1098/rsbl.2010.0920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlick-Steiner BC, Steiner FM, Konrad H, Seifert B, Christian E, Moder K, Stauffer C, Crozier RH. 2008. Specificity and transmission mosaic of ant nest-wall fungi. Proc. Natl Acad. Sci. USA 105, 940–943. ( 10.1073/pnas.0708320105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nepel M, Voglmayr H, Schönenberger J, Mayer VE. 2014. High diversity and low specificity of Chaetothyrialean fungi in carton galleries in a neotropical ant–plant association. PLoS ONE 9, e112756 ( 10.1371/journal.pone.0112756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer VE, Frederickson ME, McKey D, Blatrix R. 2014. Current issues in the evolutionary ecology of ant-plant symbioses. New Phytol. 202, 749–764. ( 10.1111/nph.12690) [DOI] [PubMed] [Google Scholar]
- 17.Little AEF, Currie CR. 2008. Black yeast symbionts compromise the efficiency of antibiotic defenses in fungus-growing ants. Ecology 89, 1216–1222. ( 10.1890/07-0815.1) [DOI] [PubMed] [Google Scholar]
- 18.Mueller UG. 2012. Symbiont recruitment versus ant-symbiont co-evolution in the attine ant-microbe symbiosis. Curr. Opin. Microbiol. 15, 269–277. ( 10.1016/j.mib.2012.03.001) [DOI] [PubMed] [Google Scholar]
- 19.Lagerheim G. 1900. Über Lasius fuliginosus (Latr.) und seine Pilzzucht. Entomol. Tidskr. 21, 17–29. [Google Scholar]
- 20.Maschwitz U, Hölldobler B. 1970. Der Kartonnestbau bei Lasius fuliginosus Latr. (Hym. Formicidae). Z. Für Vgl. Physiol. 66, 176–189. ( 10.1007/BF00297777) [DOI] [Google Scholar]
- 21.Weissflog A. 2001. Freinestbau von Ameisen (Hymenoptera, Formicidae) in der Kronenregion feuchttropischer Wälder Südostasiens. PhD thesis, Johann Wolfgang Goethe-Universität, Frankfurt am Main, Germany.
- 22.Dejean A, Solano PJ, Ayroles J, Corbara B, Orivel J. 2005. Arboreal ants build traps to capture prey. Nature 434, 973 ( 10.1038/434973a) [DOI] [PubMed] [Google Scholar]
- 23.Mayer VE, Voglmayr H. 2009. Mycelial carton galleries of Azteca brevis (Formicidae) as a multi-species network. Proc. R. Soc. B 276, 3265–3273. ( 10.1098/rspb.2009.0768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson DW, McKey D. 1993. The evolutionary ecology of symbiotic ant-plant relationships. J. Hymenopt. Res. 2, 13–83. [Google Scholar]
- 25.Heil M, McKey D. 2003. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 34, 425–453. ( 10.1146/annurev.ecolsys.34.011802.132410) [DOI] [Google Scholar]
- 26.Rosumek FB, Silveira FAO, Neves FD, Barbosa NPD, Diniz L, Oki Y, Pezzini F, Fernandes GW, Cornelissen T. 2009. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160, 537–549. ( 10.1007/s00442-009-1309-x) [DOI] [PubMed] [Google Scholar]
- 27.Nepel M, Voglmayr H, Blatrix R, Longino JT, Fiedler K, Schönenberger J, Mayer VE. 2016. Ant-cultivated Chaetothyriales in hollow stems of myrmecophytic Cecropia sp. trees—diversity and patterns. Fungal Ecol. 23, 131–140. ( 10.1016/j.funeco.2016.07.007) [DOI] [Google Scholar]
- 28.Blatrix R, Debaud S, Salas-Lopez A, Born C, Benoit L, McKey D, Atteke C, Djiéto-Lordon C. 2013. Repeated evolution of fungal cultivar specificity in independently evolved ant-plant-fungus symbioses. PLoS ONE 8, e68101 ( 10.1371/journal.pone.0068101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokolo B, Atteke C, Ibrahim B, Blatrix R. 2016. Pattern of specificity in the tripartite symbiosis between Barteria plants, ants and Chaetothyriales fungi. Symbiosis 69, 169–174. ( 10.1007/s13199-016-0402-2) [DOI] [Google Scholar]
- 30.Blatrix R, Bouamer S, Morand S, Selosse MA. 2009. Ant-plant mutualisms should be viewed as symbiotic communities. Plant Signal. Behav. 4, 554–556. ( 10.1111/j.1469-8137.2009.02793.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maschwitz U, Fiala B, Dumpert K, Hashim R, Sudhaus W. 2016. Nematode associates and bacteria in ant-tree symbioses. Symbiosis 69, 1–7. ( 10.1007/s13199-015-0367-6) [DOI] [Google Scholar]
- 32.Esquivel A, Abolafia J, Hanson P, Pinto A. 2012. A new species of nematode, Sclerorhabditis neotropicalis sp. n. (Rhabditida), associated with Azteca ants in Cecropia obtusifolia. Nematropica 42, 163–169. [Google Scholar]
- 33.Defossez E, Djiéto-Lordon C, McKey D, Selosse MA, Blatrix R. 2011. Plant-ants feed their host plant, but above all a fungal symbiont to recycle nitrogen. Proc. R. Soc. B 278, 1419–1426. ( 10.1098/rspb.2010.1884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blatrix R, Djiéto-Lordon C, Mondolot L, La Fisca P, Voglmayr H, McKey D. 2012. Plant-ants use symbiotic fungi as a food source: new insight into the nutritional ecology of ant-plant interactions. Proc. R. Soc. B 279, 3940–3947. ( 10.1098/rspb.2012.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chomicki G, Renner SS. 2015. Phylogenetics and molecular clocks reveal the repeated evolution of ant-plants after the late Miocene in Africa and the early Miocene in Australasia and the Neotropics. New Phytol. 207, 411–424. ( 10.1111/nph.13271) [DOI] [PubMed] [Google Scholar]
- 36.Chomicki G, Ward PS, Renner SS. 2015. Macroevolutionary assembly of ant/plant symbioses: Pseudomyrmex ants and their ant-housing plants in the Neotropics. Proc. R. Soc. B 282, 20152200 ( 10.1098/rspb.2015.2200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gueidan C, Villasenor CR, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F. 2008. A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud. Mycol. 61, 111–119. ( 10.3114/sim.2008.61.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddison WP, Maddison DR. 2015. Mesquite: a modular system for evolutionary analysis. Version 3.04. See http://mesquiteproject.org.
- 39.Lutzoni F, Wagner P, Reeb V, Zoller S. 2000. Integrating ambiguously aligned regions of DNA sequences in phylogenetic analyses without violating positional homology. Syst. Biol. 49, 628–651. ( 10.1080/106351500750049743) [DOI] [PubMed] [Google Scholar]
- 40.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. ( 10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 41.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE), pp. 1–8. New Orleans, LA, USA: IEEE.
- 42.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 ( 10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. ( 10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 44.Ronquist F, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. ( 10.1093/sysbio/sys029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoch CL, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl Acad. Sci. USA 109, 6241–6246. ( 10.1073/pnas.1117018109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Hoog GS, Vicente V, Caligiorne RB, Kantarcioglu S, Tintelnot K, van den Ende AHGG, Haase G. 2003. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. J. Clin. Microbiol. 41, 4767–4778. ( 10.1128/JCM.41.10.4767-4778.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estévez E, Veiga MC, Kennes C. 2005. Biodegradation of toluene by the new fungal isolates Paecilomyces variotii and Exophiala oligosperma. J. Ind. Microbiol. Biotechnol. 32, 33–37. ( 10.1007/s10295-004-0203-0) [DOI] [PubMed] [Google Scholar]
- 48.Baker CC. 2015. Complexity in mutualisms: indirect interactions with multiple parties. PhD thesis. Harvard University, Cambridge, MA, USA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: GenBank accessions KX822178-KX822578. Phylogenetic data, including alignments: TreeBase accession URL http://purl.org/phylo/treebase/phylows/study/TB2:S19475.