Abstract
Mycobacterium spp. infections are common in zebrafish kept in research facilities. These comorbidities can substantially modulate the responses of these fish to external and internal stimuli. Therefore, diagnostic tests to detect Mycobacterium spp. infections in zebrafish colonies prove essential. Here, we outline the development of quantitative simplex real-time PCR assays to detect the 3 Mycobacterium species most commonly identified in laboratory zebrafish. The assays targeted the heat-shock protein 65 gene of M. marinum, M. chelonae, and M. haemophilum. The assays are both highly specific and sensitive for fresh-frozen samples and highly specific and moderately sensitive for formalin-fixed paraffin-embedded (FFPE) samples. Two sampling techniques for FFPE samples of sagittally sectioned zebrafish were evaluated. Both paraffin cores targeting granulomas containing bacteria and scrolls from the entire fish yielded DNA of equivalent quantity and purity. The diagnostic sensitivity of cores was superior to that of scrolls for M. chelonae and M. haemophilum but not M. marinum. The assays are cost-effective and ideally suited to diagnosing common Mycobacterium spp. infections in zebrafish.
Abbreviations: Ct, cycle threshold; FFPE, formalin-fixed paraffin-embedded; qPCR, quantitative real-time polymerase chain reaction; Tm, melting temperature
Zebrafish (Danio rerio) are quickly gaining popularity in the research setting as a preclinical animal model. To remove confounding comorbidities and prevent the loss of vital resources and study animals, diagnostic and screening tests are needed to detect some of the most common and devastating infections in zebrafish: mycobacterioses. Although numerous Mycobacterium spp. can infect zebrafish, 3 species in particular are of concern due to their high morbidity or mortality or subclinical infection with high bacterial loads. M. marinum and M. haemophilum have both been documented as causing widespread outbreaks in research settings;28,29 M. chelonae infections are more common in zebrafish1,7,26,27 and usually result in low mortality but are of concern because apparently healthy (subclinically infected) fish may have extensive internal lesions with high bacterial loads. Mycobacterial infections in experimental animals also pose a health threat to laboratory personnel. Given the importance of these infections and the differences in severity of clinical disease due to mycobacterial species, it is important to develop rapid, sensitive, and specific diagnostic tests for these 3 pathogens. Molecular diagnostic methods are now the most practical and efficient approach to detect and identify mycobacteria to the species level, because many species are difficult to grow in culture and because biochemical tests are often not informative.33 These PCR methods may involve amplification of DNA from fresh, frozen, or ethanol-preserved tissues and target areas of high discriminatory power, such as hsp65, rpoB, and the 16S–23S internal transcriber spacer. This approach has been used with piscine Mycobacterium species that are difficult to culture, such as M. haemophilum in zebrafish and M. triplex-like organisms in rockfish, swordtails, and mollies.19,27-29,37 Alternatively, species and strain identifications can be rapidly and accurately obtained by using PCR tests of bacteria isolated in culture.37 In zebrafish, PCR testing for mycobacteria by using formalin-fixed or paraffin-embedded (or both) material is particularly useful, because histopathology is generally the primary diagnostic method used for this species, and fresh tissues and cultures are often unavailable.9,12,17
The purpose of the current study was to develop simplex (that is, using a single primer or probe set to detect a particular species), real-time quantitative PCR (qPCR) assays for each of the aforementioned mycobacterial species of importance to zebrafish for 3 types of samples: bacterial isolates, fresh-frozen fish, and formalin-fixed paraffin-embedded (FFPE) fish. The resulting assays have aided in the identification of mycobacterial outbreaks and their specific causes. Furthermore, the assays will serve as screening tools to develop and maintain SPF zebrafish lines.8
Materials and Methods
Bacterial strains and cultures.
Mycobacterial cultures used for experimental infection were prepared as described following. Three isolates each of M. marinum and M. chelonae (Figure 1) were obtained from the Kent laboratory at Oregon State University (Corvallis, OR). Specifically, 2 isolates of M. marinum (MmE and MmW) were obtained from zebrafish from outbreaks at 2 large research facilities, and the third (OSU214) was isolated from hybrid striped bass and identical to isolates from zebrafish based on pulsed-field gel electrophoresis.15,26 The 3 M. chelonae isolates originated from zebrafish with low-level mortality.29 The single isolate of M. haemophilum was obtained through ATCC (no. 29548, Vienna, VA). The M. marinum and M. chelonae isolates were streaked onto Middlebrook 7H10 media and incubated for 14 d at 32 °C; the M. haemophilum isolate was streaked on Middlebrook 7H10 media enhanced with hemin and incubated for 28 d at 32 °C.32 Cultured colonies from each isolate were transferred to appropriate broth media by using loop inoculation to generate serial dilutions. Fresh culture material was placed in sterile PBS to obtain a stock solution of approximately 3 × 108 cells per mL by using the MacFarland optical density of 1.0 as a reference.16 A 1:10 dilution of these stocks (3 × 107) was used to infect fish by injecting 10 μL (approximately 3 × 105 organisms per fish). Control isolates (Figure 1) were as follows: attenuated BCG Pasteur from the Institute Pasteur (Paris, France) and K10 M. avium subsp. paratuberculosis from Raul Barletta (University of Nebraska, Lincoln, NE) were provided by the laboratory of Luiz Bermudez at Oregon State University; Streptococcus inia, Nocardia nova, Pasteurella multocida, and Corynebacterium pseudotuberculosis isolated from diagnostic samples and phenotypically characterized were provided by the Veterinary Diagnostic Laboratory at Oregon State University.25,31
Figure 1.
Bacterial isolates used to develop qPCR assays for mycobacteria commonly diagnosed in zebrafish research colonies.
Zebrafish.
Adult wild-type zebrafish of both sexes were acquired from the Sinnhuber Aquatic Research Laboratory (Oregon State University), a facility that is SPF for Pseudoloma neurophilia and has a very low background infection with M. chelonae.8 The AB/5D strain was used for infection with M. marinum and M. haemophilum, the TU/5D strain was used for infection with M. chelonae. Both the AB and 5D strains are SPF for Pseudoloma neurophilia and M. marinum, M. haemophilum, and M. chelonae. The TU strain is not certified SPF, but this colony of TU/5D crosses has repeatedly tested negative for those pathogens. The fish were maintained in a Biosafety Level 2 facility in flow-through tanks according to an Animal Care and Use Protocol approved by the IACUC at Oregon State University. For inoculation, zebrafish were anesthetized with 100 mg buffered 1−1 tricaine methanosulfonate (MS222) and injected with either 10 μL PBS (sham inoculation) or prepared M. marinum (OSU214), M. chelonae (H1E1), and M. haemophilum inocula by using aseptic technique. Zebrafish were maintained at 25 to 27 °C with sufficient aeration and a 14:10-h light:dark photoperiod for 12 wk to allow for infection to develop. Dead fish were collected daily, and all surviving zebrafish were euthanized at the end of the 12-wk period. Euthanized zebrafish were either fresh-frozen (–80 °C) or fixed in 10% neutral-buffered formalin for 7 d followed by decalcification in CalExII (ThermoFisher Scientific, Waltham, MA) for 48 h. A total of 15 fish from each isolate per species were fresh-frozen, and another 15 from each isolate were fixed for paraffin embedding, although only 6 were used in the experiments. Fixed zebrafish were held in a 70% ethanol solution until processing into paraffin blocks for a maximum of 8 wk at the Veterinary Diagnostic Laboratory (Oregon State University). For evaluation by light microscopy, 3-μM sections were stained with hematoxylin and eosin (ThermoFisher Scientific) or Kinyoun acid-fast stain (Kinyoun carbol fuchsin, VWR, Radnor, PA) according to standard protocols and operating procedures.2
Primer and probe design.
Primers were designed based on published sequences of the bacterial heat-shock protein 65 gene, which is highly conserved within the Mycobacterium genus but variable enough to distinguish between Mycobacterium species.10 Sequences of the HSP65 gene were obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and analyzed by using Primer Express 3.0 Software to generate primer and probe sets.21 Primer and probes were: M. marinum (GenBank accession no., AF547855 #6) MmForward, 5′ CAA CCC GCT CGG TCT GAA 3′ (melting temperature [Tm], 59 °C; GC content, 61%); MmReverse, 5′ CGA CCT CTT TGG CCG ACT T 3′ (Tm, 59 °C; GC content, 58%); and MmProbe, 5′ TCA CCG AGA CCT TGC 3′ (Tm 69 °C; GC content, 60%); M. chelonae (DQ866784 #31) McForward, 5′ AAG GAA GTT GCC AAG AAG ACT GA 3′ (Tm 58 °C; GC content, 43%); McReverse, 5′ CAG AGC CTG GGC AAG CA 3′ (Tm, 58 °C; GC content, 65%); and McProbe, 5′ ACG GCA CTA CTA CCG C 3′ (Tm, 69 °C; GC content, 63%); and M. haemophilum (GQ245967#2) MhForward, 5′ GTT AAG GTG GCG TTG GAA GCT 3′ (Tm, 58 °C; GC content, 50%); MhReverse, 5′ TCC AGC CCG GAG TTG AAG 3′ (Tm, 58 °C; GC content, 61%); and MhProbe, 5′ CGC TGA AGC AGA TCG 3′ (Tm, 69 °C; GC content, 60%)
Primer and probe sets were analyzed for sequence similarity to other Mycobacterium species and were used in a BLAST search to identify sequence similarity across all bacterial genera. In addition, each primer and probe set was examined for sequence similarity with the other sets. Selected sets had a low penalty score (Primer Express 3.0 Software) and high specificity as determined by sequence similarity analysis (Table 1).
Table 1.
Sequence homology between primers and probes to mycobacterial heat-shock protein 65
| MmForward | MmReverse | MmProbe | |
| MmForward | NA | 5/18 (27.8%) | 4/15 (26.7%) |
| MmReverse | NA | NA | 5/15 (33.3%) |
| MmProbe | NA | NA | NA |
| McForward |
McReverse |
McProbe |
|
| McForward | NA | 4/17 (23.5%) | 4/16 (25.0%) |
| McReverse | NA | NA | 7/16 (43.7%) |
| McProbe | NA | NA | NA |
| MhForward |
MhReverse |
MhProbe |
|
| MhForward | NA | 3/18 (16.7%) | 2/15 (13.3%) |
| MhReverse | NA | NA | 3/15 (20.0%) |
| MhProbe | NA | NA | NA |
Mm, M. marinum OSU214; Mc, M. chelonae H1E1; Mh, M. haemophilum; NA, not applicable
Data are given as the number of base pair matches between the 2 sequences / the number of nucleotides present in the shorter of the 2 sequences, starting at the 3’ end (percentage of homology)
Sample preparation and DNA extraction.
Aliquoted isolate samples were centrifuged at 6000 × g for 5 min, the supernatant removed, and the pellet resuspended in 200 μL PBS; 20 μL lysozyme (10 mg/mL; ThermoFisher Scientific) was added to each sample to optimize the assays and improve diagnostic yield. After incubation at 37 °C for 60 min, the DNA in 50 μL of the solution was extracted (MagMAX Total Nucleic Acid Isolation Kit, Life Technologies, Grand Island, NY) according to the manufacturer's instructions. Different concentrations of primers and probes used in the Master Mix were tested to optimize diagnostic yield. For primers, 0.2, 0.4, 0.6, and 0.8 μM concentrations were tested; for probes, 120 and 240 nM concentrations were tested.
Fresh-frozen samples of whole fish were thawed at room temperature and minced on individual culture dishes by using new scalpel blades for each fish to prevent cross-contamination. Screw-top centrifuge tubes containing 0.1 g of tissue sample and zirconia–silica beads (0.1 mm, Biospec Products, Bartlesville, OK) in 1 mL PBS were placed on a Mini-BeadBeater 16 (Biospec Products) for 3 min. After centrifugation at 1000 × g for 1 min, the supernatant was used for DNA extraction as described in the preceding paragraph.
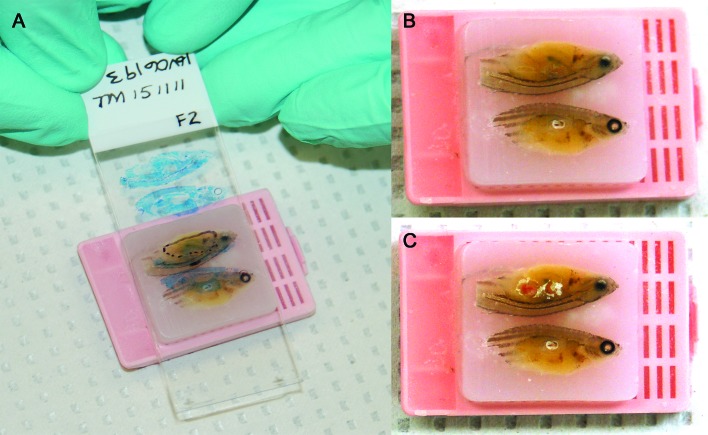
Prior to testing of FFPE samples, their respective corresponding slides were evaluated to locate lesions for core sampling. If the sample quality was poor (due to advanced autolysis as determined by a board-certified veterinary pathologist [CVL]), that sample was omitted from the test run. Scrolls of embedded zebrafish were generated by sectioning paraffin blocks (thickness, 5 µm; model 820 II rotary microtome, Reichert–Jung, Depew, NY); sections were immediately transferred into sterile microfuge tubes. Between each block, the microtome blade was changed and the microtome wiped down with xylene followed by 90% ethanol. Three negative (that is, paraffin only) blocks were sampled after every 5 test blocks for M. chelonae and M. haemophilum and after every 3 test blocks for M. marinum to prevent and allow for testing for cross-contamination.17 To generate cores, acid-fast–stained slides of infected fish were analyzed to identify granulomas containing mycobacterial organisms in situ. Lesions were marked on each slide and matched to the location in the paraffin-embedded zebrafish (Figure 2). Core samples were collected by using a new, sterile 16-gauge injection needle for each fish. DNA was extracted by using the RecoverAll Total Nucleic Acid Kit for FFPE (Life Technologies) according to manufacturer's instructions. For optimal yield, the protease digestion step was performed overnight (approximately 20 h) at 50 °C.
Figure 2.
(A) Paraffin block of an embedded zebrafish diagnosed with mycobacteriosis according to histologic examination. The corresponding acid-fast–stained microscopic slide with marked-up mycobacterial granulomas is overlaid to select specific areas for coring. Same paraffin block as in panel A but (B) before and (C) after collection of core samples. Images were modified to adjust white balance.
Real-time qPCR assays.
Isolates.
Samples were PCR-amplified (model 7500 Fast Real-Time PCR System, Applied Biosystems, Foster City, CA) by using an initial uracil-N glycosylase incubation cycle of 50 °C for 2 min, followed by an Amplitaq (Life Technologies, Grand Island, NY) activation cycle of 95 °C for 10 min and 40 cycles of denaturation at 95 °C for 15 s with an annealing–extension step at 57 °C for 1 min. PCR products were visualized by using GelRed (Biotium, Hayward, CA) in a 2.5% agarose gel to determine specificity and to obtain samples for sequencing (Center for Genome Research and Biocomputing, Oregon State University) to confirm that the desired product was obtained.
To determine analytical specificity as well as reproducibility and repeatability of the PCR assays, both inter- and intra-assay comparison runs were performed for all simplex assays using serial dilutions of isolate samples of each test species along with the control isolates (attenuated BCG and K10; Streptococcus iniae, Nocardia nova, Pasteurella multocida, and Corynebacterium pseudotuberculosis). When multiple isolates were available for a species, a single isolate was chosen on which to perform intra-assay comparisons. Serial dilutions ranging from 101 to 108 (see following) were tested 4 times. The mean cycle threshold (Ct) values for each dilution were plotted on a line graph to determine linear regression (R2), with an R2 value greater than 0.95 being ideal (Excel 2010, Microsoft, Redmond, WA). The coefficient of variation was expressed as a percentage and is a measure of repeatability or precision; CV values greater than 4% are considered to be too variable, or imprecise, to be useful. For interassay comparison, all isolates were tested 4 times (except for M. haemophilum, for which only one isolate was available and therefore could not be compared with other isolates), and Ct values plotted on a line graph to determine linear regression with an R2 value greater than 0.95 being ideal.
The analytical sensitivity of the simplex qPCR assays, as defined by limit of detection, was determined by using 10-fold serial dilutions made from stock solutions of eachisolate. The stock solutions were generated by using a MacFarland optical density of 1, which reflects an approximate bacterial cell density of 3 × 108/mL, as a starting point. The 1:10-diluted solutions of these stocks (3 × 107/mL) were used to infect fish with 3 × 105 organisms by the injection of 10 μL. More dilute solutions (specifically 3 × 104, 3 × 103, and 3 × 102 cells/mL) were plated to confirm that the expected number of colony-forming units was obtained. All dilutions of an isolate were amplified on the same plate in the same qPCR run to determine the lowest number of colony-forming units detectable.
Fresh-frozen samples.
Once the analytical specificity–sensitivity and repeatability–reproducibility of the assays were evaluated by using bacterial isolates and no cross-reactivity identified, the assays were used on fresh frozen tissue samples as described for isolates. To assess specificity and sensitivity, 9 fresh-frozen fish per species were used, taking 3 from each isolate for M. marinum and M. chelonae, given that 3 isolates were available for these species. When testing repeatability and reproducibility, 4 fish per isolate were tested. The same thermal cycling parameters were used as for the isolates.
FFPE samples.
After the specificity–sensitivity and repeatability–reproducibility of the simplex assays were established for fresh-frozen samples, each simplex assay was tested on FFPE samples on both scroll and matched core samples for each of the 3 mycobacterial species. The same thermal cycling parameters were used as described for the isolates, except that 50 cycles of the denaturation and annealing–extension steps were performed to increase yield. Both the purity and concentration of the DNA isolated from FFPE samples were determined by UV spectrometry (Nandrop 2000, ThermoScientific; Table 2).
Table 2.
Paraffin-embedded zebrafish and control blocks: granuloma distribution and mycobacterial load according to microscopy; DNA quantity and quality; and qPCR results
| DNA |
||||||||||
| Concentration (ng/μL) |
Purityb (260:280 nm) |
qPCR |
||||||||
| Isolate | Fish no. | Internal fish no. | Lesions | Categorya | scrolls | cores | scrolls | cores | scrolls | cores |
| M. marinum OSU214 | 1 | R12-MVT-73 | Large granulomas in kidney, spleen | 2 | 210.7 | 230.1 | 1.68 | 1.83 | + | + |
| 2 | R12-MVT-70 | Numerous acid-fast bacilli scattered in spleen, adjacent to swim bladder; poorly preserved granulomas adjacent to swim bladder | 2 | 126.7 | 170.2 | 1.83 | 1.75 | – | – | |
| 3 | R12-MVT-67 | Numerous acid-fast bacilli scattered in spleen, adjacent to swim bladder; focal granuloma in pericardium | 2 | 169.4 | 185.7 | 1.76 | 1.73 | + | – | |
| N1d | Not applicable | NA | 141.1 | NA | 1.87 | NA | – | NA | ||
| N2 | Not applicable | NA | 119.7 | NA | 1.85 | NA | – | NA | ||
| N3 | Not applicable | NA | 182.5 | NA | 1.73 | NA | – | NA | ||
| 4 | R12-MVT-64 | Numerous large granulomas and scattered acid-fast bacilli in ovaries, kidney, spleen, liver and pericardium | 3 | 160.8 | 228.3 | 1.83 | 1.69 | + | + | |
| 5 | R12-MVT-63 | Numerous large granulomas and scattered acid-fast bacilli in spleen, kidney, liver, ovaries and adjacent to swim bladder | 3 | 178.0 | 292.9 | 1.75 | 1.74 | + | – | |
| 6 | R12-MVT-61 | Very few (~10-20) acid-fast bacilli scattered in spleen, ovary and liver; one discrete granuloma dorsal to ovaries | 1 | NA | 218.2 | NA | 1.65 | NA | – | |
| M. chelonae H1E1 | 7 | 1 | 3 or 4 large granulomas in ovary and scattered acid-fast bacilli in ovary | 2 | 1.88 | 1.82 | 139.7 | 163.7 | – | – |
| 8 | 2 | 3 or 4 large granulomas in ovary | 2 | 1.79 | 1.71 | 176.5 | 259.5 | – | + | |
| 9 | 5 | Lining swim bladder | 1 | 1.83 | 1.81 | 151.4 | 156.6 | – | + | |
| N1c | Not applicable | NA | 1.73 | NA | 123.7 | NA | – | NA | ||
| N2 | Not applicable | NA | 1.75 | NA | 127.1 | NA | – | NA | ||
| N3 | Not applicable | NA | 1.71 | NA | 133.6 | NA | – | NA | ||
| 10 | 6 | Large granulomas in ovary and scattered acid-fast bacilli in ovary | 2 | 1.83 | 1.69 | 169.8 | 449.1 | – | + | |
| 11 | 7 | Lining swim bladder | 1 | 1.85 | 1.77 | 143.5 | 198.3 | + | – | |
| 12 | 9 | Focal granuloma adjacent to swim bladder | 1 | 1.86 | 1.81 | 154.0 | 148.6 | – | + | |
| 13 | 11 | Focal granuloma adjacent to swim bladder | 1 | NA | NA | NA | NA | NA | NA | |
| 14 | 12 | Lining swim bladder | 1 | NA | NA | NA | NA | NA | NA | |
| M. haemophilum | 15 | 1 | Large granulomas adjacent to swim bladder; kidney | 2 | 136.9 | 264.9 | 1.89 | 1.68 | – | + |
| 16 | 2 | Large granulomas adjacent to swim bladder; kidney; liver | 3 | 138.9 | 189.0 | 1.80 | 1.71 | – | – | |
| 17 | 3 | Large granulomas in liver; kidney | 2 | 148.7 | 739.0 | 1.79 | 1.72 | NA | NA | |
| 18 | 4 | Large granulomas adjacent to swim bladder; kidney | 2 | 125.8 | 203.5 | 1.83 | 1.72 | NA | NA | |
| 19 | 5 | Numerous large granulomas adjacent to swim bladder; kidney; liver | 3 | 168.2 | 180.9 | 1.78 | 1.71 | NA | NA | |
| 20 | 6 | Indiscrete granulomas; lymphoplasmacytic inflammation in kidney; liver | 1 | 123.7 | 141.1 | 1.82 | 1.79 | NA | NA | |
| 21 | 7 | Large granulomas in spleen, kidney, adjacent to swim bladder | 3 | 122.7 | 197.3 | 1.82 | 1.73 | NA | NA | |
| 22 | 8 | Large granulomas in kidney, liver, adjacent to swim bladder | 3 | 125.6 | 297.0 | 1.81 | 1.66 | NA | NA | |
| 23 | 9 | Large granulomas adjacent to swim bladder, pericardium, kidney | 3 | 127.5 | 142.9 | 1.91 | 1.78 | + | + | |
| 24 | 10 | Large granulomas in kidney, adjacent to swim bladder, pericardium | 2 | 142.4 | 166.0 | 1.76 | 1.74 | – | + | |
| N1 | Not applicable | NA | 136.9 | NA | 1.86 | NA | – | NA | ||
| N2 | Not applicable | NA | 134.3 | NA | 1.81 | NA | – | NA | ||
| N3 | Not applicable | NA | 124.7 | NA | 1.79 | NA | – | NA | ||
| 25 | 11 | Lymphoplasmacytic inflammation in pericardium, kidney; smaller granulomas adjacent to swim bladder | 1, 2 | 145.6 | 198.6 | 1.76 | 1.71 | + | – | |
| 26 | 12 | Large granuloma adjacent to swim bladder, kidney; lymphoplasmacytic inflammation in pericardium | 3 | 136.2 | 117.7 | 1.87 | 1.89 | + | + | |
| 27 | 13 | Lymphoplasmacytic inflammation in kidney, adjacent to swim bladder ± pericardium | 1 | NA | NA | NA | NA | NA | NA | |
| 28 | 14 | Lymphoplasmacytic inflammation adjacent to swim bladder, kidney | 1 | NA | NA | NA | NA | NA | NA | |
| 29 | 15 | Numerous granulomas in kidney, liver, adjacent to swim bladder; lymphoplasmacytic inflammation in pericardium | 3 | NA | NA | NA | NA | NA | NA | |
aCategory denotes severity of infection: 1, mild; 2, moderate; 3, severe
bPurity of DNA preparation according to the 260:280-nm absorbance ratio of the sample
cN denotes an empty paraffin block used between sectioning specimens for scrolls to prevent and control for cross-contamination
Statistics.
Detection limits (that is, the lowest number of colony-forming units that yielded a positive PCR result) were calculated for all mycobacterial isolates under investigation. Reproducibility and repeatability were calculated (Excel 2010, Microsoft) as the mean, 1 SD, and coefficient of variance by using the Ct values of serial dilutions of representative isolates of each mycobacterial species. Ct values were tabulated for a minimum of 3 replicates. Diagnostic specificity and sensitivity were calculated (Excel 2010, Microsoft) on samples from experimentally infected zebrafish by using the current ‘gold standard’ of diagnosis: detection of acid-fast bacteria in granulomas in situ. Diagnostic and analytical sensitivity were calculated as the number of true positives divided by the number of all positives, whereby ‘positive’ refers to a samples or dilution, respectively. Diagnostic and analytical specificity was calculated as number of true negatives divided by all negatives, whereby ‘negative’ refers to a sample or dilution, respectively.
Results
Histopathology.
Findings from microscopic examination of tissue samples stained with hematoxylin and eosin or acid-fast stain are detailed in Table 2. Zebrafish infected with M. marinum (n = 6) had few, scattered acid-fast bacilli; acid-fast bacteria were present in the spleen of all zebrafish. Other common body sites were kidney, liver, and ovaries and within or adjacent to the swim bladder. Infection with M. chelonae (n =7) presented in about half of the fish with large granulomas in the ovary or adjacent to the swim bladder; in the other half, acid-fast bacteria lined the lumen of the swim bladder. M. haemophilum-infected zebrafish (n = 14) had large granulomas, which were located in the kidney in all fish as well as (in decreasing prevalence) adjacent to the swim bladder, in the liver, or in the pericardium.
qPCR analysis of bacterial isolates: analytical specificity, sensitivity, and reproducibility.
Analytical specificity was confirmed for all qPCR assays. Each assay proved to be specific and yielded the expected PCR amplicon for the tested mycobacterial species (data not shown). None of the simplex assays yielded any amplification products for the control isolates (attenuated BCG and K10; Streptococcus iniae, Nocardia nova, Pasteurella multocida, and Corynebacterium pseudotuberculosis). An assay was determined specific when only isolates of the particular Mycobacterium species yielded an amplification product of the expected size and when the sequence of the amplification product matched the reference genome by at least 99%.
Analytical sensitivity, defined as the limit of detection of 10-fold serial dilutions made from stock solutions (3 × 108/mL) of each Mycobacterium spp. isolate, ranged from 3000 to 300,000 (Table 3). For M. marinum isolates, detection limits differed by as much as 10-fold. The M. chelonae isolates showed a maximum of a 100-fold difference in the detection limit when the same dilution was used for each test species. The addition of lysozyme decreased (improved) the Ct values from approximately 36 to approximately 31. The optimal primer and probe concentrations were 0.4 µm and 120 nM, respectively.
Table 3.
Detection limits of simplex PCR assays for mycobacterial isolates
| Isolate | Lowest dilution at which a signal was detectablea | CFUb |
| M. marinum MmE | 105 | 30,000 |
| M. marinum MmW | 105 | 30,000 |
| M. marinum OSU214 | 106 | 300,000 |
| M. chelonae H1E1 | 104 | 3000 |
| M. chelonae H1E2 | 106 | 300,000 |
| M. chelonae H1E3 | 104 | 3000 |
| M. haemophilum | 105 | 30,000 |
Based on serial 10-fold dilutions, starting at 108 (with an estimated 3 × 108 cells per mL).
Number of colony-forming units obtained after plating of 100 μL.
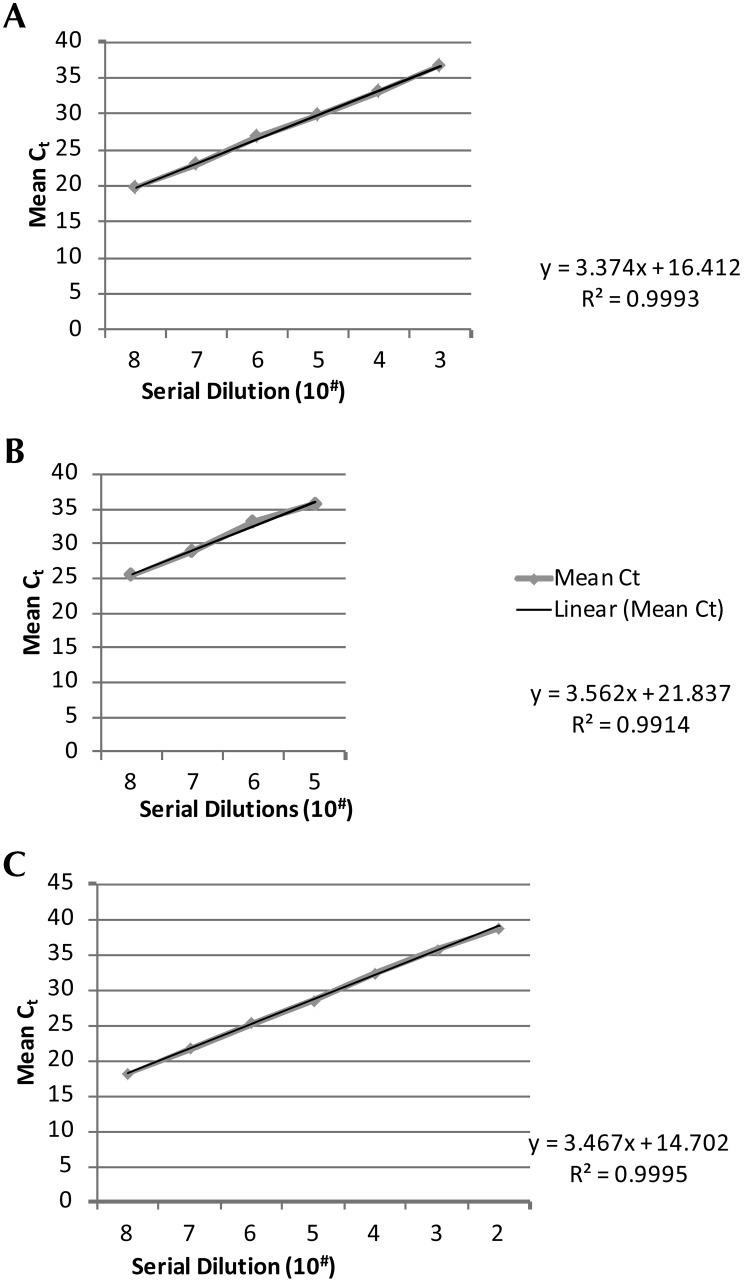
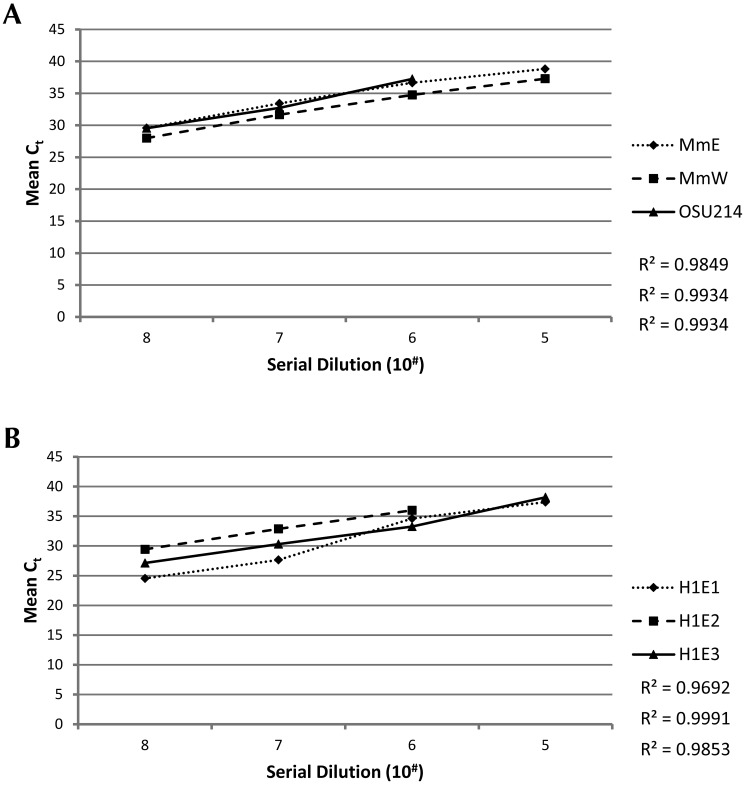
To determine analytical reproducibility and repeatability, both intra- and interstrain (that is, isolate) comparison runs were performed by using serial dilutions of multiple isolates wherever possible; only a single isolate of M. hemophilum was available. For intrastrain comparison runs, serial 10-fold dilutions of individual isolates for each Mycobacterium spp. were made and then run on the same plate, with 4 replicates of each dilution. The qPCR results for the intrastrain analysis are provided in Table 4 and Figure 3. For interstrain analysis, serial dilutions were created for each isolate of M. marinum and M. chelonae to compare the sensitivity of each simplex assay for each isolate at various concentrations. The qPCR results are provided in Table 5 and Figure 4.
Table 4.
Intraassay comparison of qPCR assays for mycobacteria
| Replicate number |
|||||||
| Dilution | 1 | 2 | 3 | 4 | Mean | 1 SD | CV (%) |
| M. marinum (mME) | |||||||
| 102 | not done | 39.40 | not done | not done | |||
| 103 | not done | not done | 37.41 | 36.00 | 36.70 | 0.99 | 2.72 |
| 104 | 32.29 | 33.90 | 33.75 | 32.68 | 33.15 | 0.79 | 2.39 |
| 105 | 29.98 | 29.94 | 29.56 | 29.73 | 29.80 | 0.19 | 0.66 |
| 106 | 26.93 | 26.24 | 27.91 | 26.34 | 26.86 | 0.76 | 2.86 |
| 107 | 23.02 | 22.97 | 23.63 | 22.58 | 23.05 | 0.43 | 1.89 |
| 108 | 19.89 | 20.09 | 19.69 | 19.27 | 19.73 | 0.34 | 1.77 |
| M. chelonae (H1E1) | |||||||
| 105 | 36.03 | 35.57 | 36.00 | 35.21 | 35.70 | 0.38 | 1.09 |
| 106 | 33.54 | 32.85 | 33.10 | 33.04 | 33.13 | 0.28 | 0.87 |
| 107 | 29.31 | 28.39 | 29.19 | 28.50 | 28.85 | 0.46 | 1.61 |
| 108 | 25.19 | 25.10 | 25.48 | not done | 25.26 | 0.19 | 0.78 |
| M. haemophilum | |||||||
| 102 | not done | 39.58 | not done | 38.70 | 38.70 | 0.62 | 1.61 |
| 103 | 35.44 | 36.44 | 34.95 | 35.76 | 35.65 | 0.62 | 1.75 |
| 104 | 32.57 | 32.00 | 31.69 | 32.74 | 32.25 | 0.49 | 1.52 |
| 105 | 28.79 | 28.38 | 28.45 | 28.35 | 28.49 | 0.20 | 0.71 |
| 106 | 25.77 | 25.61 | 24.45 | 24.96 | 25.20 | 0.60 | 2.40 |
| 107 | 21.71 | 21.85 | 21.32 | 21.65 | 21.63 | 0.22 | 1.03 |
| 108 | 17.45 | 18.02 | 18.69 | 17.98 | 18.04 | 0.50 | 2.82 |
Data are given as the Ct value.
Figure 3.
Intraassay comparisons for mycobacterial simplex qPCR assays. Shown are qPCR cycle threshold (Ct) values (y axis) for serial 10-fold dilutions of the titer (CFU; x axis) of single isolates. For each isolate, all dilutions were run in 4 replicates on the same plate. All assays showed high reproducibility and repeatability, with r values that exceeded 0.99. The calculated formulas for each qPCR assay are provided in the respective graph. Note the different lengths of the log scale for individual qPCR assays and mycobacterial species. Sensitivity was highest for M. haemophilum and lowest for M. chelonae. (A) M. marinum, MmE isolate. (B) M. chelonae, H1E1 isolate. (C) M. haemophilum.
Table 5.
Sensitivity of qPCR assay across isolates
| Isolate |
|||
| Dilution | MmE | MmW | OSU214 |
| M. marinum | |||
| 108 | 29.58 | 28.00 | 29.55 |
| 107 | 33.44 | 31.67 | 32.72 |
| 106 | 36.66 | 34.74 | 37.25 |
| 105 | 38.82 | 37.28 | not done |
| M. chelonae | H1E1 |
H1E2 |
H1E3 |
| 108 | 24.54 | 29.44 | 27.14 |
| 107 | 27.64 | 32.89 | 30.32 |
| 106 | 34.62 | 36.00 | 33.28 |
| 105 | 37.38 | not done | 38.19 |
Data are given as the Ct value.
Figure 4.
Sensitivity of simplex qPCR assays across M. marinum and M. chelonae isolates. Shown are qPCR Ct values (y axis) for serial 10-fold dilutions of titers (CFU; x axis) of 3 isolates per mycobacterial species. For each isolate, all dilutions were run in 4 replicates on the same plate and are depicted as decreasing concentrations. (A). The sensitivity of the M. marinum qPCR assay was nearly identical among all isolates: MmE (dotted line and diamonds), MmW (dashed line and squares), and OSU214 (solid line and triangles). (B) The sensitivity of the M. chelonae qPCR assay varied slightly among isolates from testes (listed from least to most sensitive): H1E2 (dashed line and squares), H1E3 (dotted line and triangles), and H1E1 (solid line and diamonds).
qPCR analysis of fresh-frozen samples.
The assays detected the respective organism in 100% of the infected fish. Of noteworthy mention, the Ct values were lower (that is, bacterial loads were higher) with chronic infections (infected fish that survived until euthanized, approximately 3 wk on average) than in zebrafish that died within days of injection (data not shown). For example, for fresh-frozen fish infected with M. chelonae, early deaths led to Ct values as high as 36, whereas chronic infections (approximately 8 wk) resulted in a Ct value as low as 31.
qPCR analysis of FFPE samples: scrolls compared with cores.
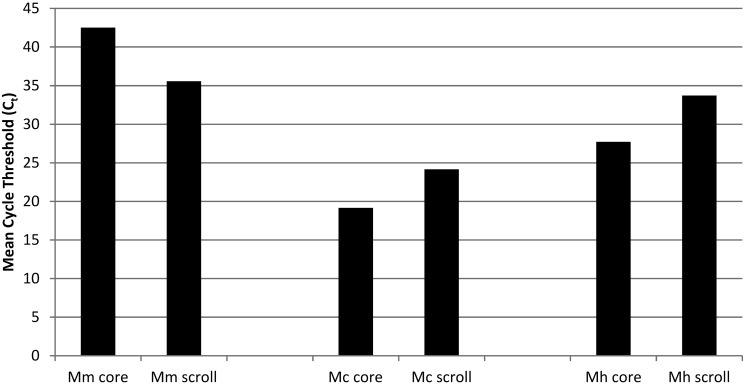
The DNA quantity and quality and the results from the 3 PCR assays are provided in Table 2. The same 6 test samples for each Mycobacterium spp. were evaluated by using both the scroll and core sampling technique. None of the control samples taken from empty paraffin blocks interspersed between infected samples tested positive in any of the simplex qPCR assays (Table 2); thus diagnostic specificity was 100% for both scrolls and cores. In general, the yield from paraffin-embedded material was fairly low for each qPCR assay, with the lowest levels obtained from M. chelonae-infected fish (Figure 5). In core samples, each mycobacterial infection was detected in at least some of the fish, yielding a diagnostic sensitivity of 33.3% (2 of 6) for M. marinum, 50% (3 of 6) for M. chelonae, and66.7% (4 of 6) for M. haemophilum. The diagnostic sensitivity for detection of mycobacterial infections in scrolls was slightly more variable between species: M. marinum, 66.7% (4 of 6); M. chelonae, 16.7% (1 of 6), and M. haemophilum, 50% (3 of 6).
Figure 5.
Mean Ct values of cores compared with scrolls of FFPE fish experimentally infected with Mycobacterium spp. Shown are mean Ct values for cores compared with scrolls obtained by respective simplex quantitative PCR assays for M. marinum (OSU214), M. chelonae (H1E1), and M. haemophilum.
Discussion
The primary goal of this project was to develop simplex real-time qPCR assays to detect the 3 most problematic mycobacterial species for screening and epidemiologic purposes in zebrafish husbandry and research. A secondary goal was to optimize the assays for a variety of specimen types—bacterial isolates, fresh-frozen zebrafish, and FFPE zebrafish—from both tissue sections (scrolls) and by targeting lesions in embedded zebrafish (cores).
The developed simplex qPCR assays we described here are specific for the examined mycobacterial species (M. marinum, M. chelonae and M. haemophilum) because none generated amplification products from other bacterial isolates of species within and outside of their genus. For bacterial isolates, the limit of detection varied among isolates within a species, likely due to variant genetic variation in M. marinum and M. chelonae hsp65 sequences. Nonetheless, an acceptable limit of detection ranging from 30 to 3000 CFU was obtained regardless of the isolate or species being tested. Mycobacteria, in general, are known to have a high limit of detection in PCR assays (that is, require more organisms to be detected), given that robust cell walls protect their DNA.5,14 Even with a lysozyme step added prior to nucleic acid isolation, it is difficult to obtain a large quantity of DNA.17 Therefore, the number of colony-forming units required to detect mycobacteria by using PCR assays is greater than those documented for other bacteria.5 Alternatively, perhaps the calculation of CFU counts by using the MacFarland method overestimated the bacteria present in the stock solution, such that sensitivity is actually higher than reported.
For fresh-frozen samples, each simplex assay achieved 100% analytical sensitivity, because we obtained positive results from all of the experimentally infected fish. The Ct values differed with chronicity of infection, such that lower Ct values were seen with chronic (established) infections, averaging about 5 cycles fewer than those of acute infection (that is, Ct of 31 instead of 36). This finding suggests a greater number of bacterial organisms in chronic infections, whereas fewer organisms are present in acute infections before substantial bacterial replication and dissemination to organs. Alternatively, mortalities that occurred within days after inoculation might have resulted from stress associated with anesthesia and intraperitoneal injection, even though all fish were handled with great care. These findings are of importance with regard to the duration of quarantine and timing of testing of quarantined fish. False negative results may occur when testing fish that die shortly after arriving at a facility and may warrant later testing (that is, in 2 to 3 w) of any fish cohoused with the early mortalities.
Using paraffin scrolls, one group obtained PCR product from 75% and 88% of M. chelonae- and M. marinum-infected zebrafish, respectively.17 In addition, although these PCR tests were directed toward heat-shock protein DNA, the assay was not quantitative. We found somewhat more variable results, with positive samples ranging from 33.3% to 66.7% for core samples and 16% to 80% for scrolls. Detection of M. haemophilum was the most reliable of the 3 examined assays. A previous protocol used a different nucleic acid isolation kit and had different cycling parameters for a conventional PCR assay, as compared with the real-time PCR assay we developed in the current project.17 These differences might account for the varying percentages of detection. Moreover, the primers and probes used in our assays were created initially in the hope of developing a multiplex PCR assay (data not shown), which places stricter requirements on primers and probes than does a simplex PCR assay. The percentage homology between primer and probe sets is lower for multiplex than simplex PCR assays, to avoid the production of undesired PCR products such as primer dimers.
Decreased diagnostic sensitivity is a known challenge for qPCR tests on FFPE samples and most likely is due to DNA degradation during the fixation process.3 A previous study found that fixative type, time in fixative, and mycobacterial species showed no statistical relationship with efficacy.17 In that study, more than half the fish held for 45 d in fixative before being processed into paraffin were positive. However, some known positives held for only 3 d in fixative yielded a negative PCR result. Tissue decalcification can similarly negatively affect DNA quantity and quality, but this step is unavoidable when preparing whole zebrafish for histologic examination. The effect is highly variable and depends on the specific acid used, with EDTA and formic acid being least detrimental.23 The decalcification solution applied to fixed zebrafish in the current study uses formic acid. Other factors that can reduce the sensitivity of PCR-based assays are the binding of primers or probes to the DNA of other bacteria present in the samples (such as the gut and skin microbiome) and the copy number of the targeted gene. None of the runs of our assays yielded primer dimers or nontarget amplification products. Because bacterial 16S rRNA codon copy numbers typically range from 1 to 15, targeting the 16S–23S rRNA internal transcribed spacer has the potential to increase sensitivity when determining bacterial loads.35 However, most of the mycobacterial species examined to date, including M. marinum, contain only a single copy of the 16S rRNA gene (ribosomal RNA database at the University of Michigan).6 Assays targeting the 16S rRNA gene for speciation generally rely on posthoc analyses, such as sequencing or capillary gel electrophoresis, which are unlikely to be available to diagnostic units and increase the cost of testing.4 A Mycobacterium spp.-general PCR assay will differentiate FFPE samples that are negative with the specific tests and contain other species of mycobacteria from those that are negative due to inadequate, degraded, or absent DNA in the sample.20
In an effort to improve DNA yield, we tested different sampling techniques—scrolls compared with cores—but neither technique was superior. One reason for this finding may be the manner in which different Mycobacterium spp. distribute throughout infected zebrafish. Both M. marinum and M. haemophilum infections in zebrafish caused widespread granuloma formation as previously reported.24,28 In contrast, M. chelonae infection in zebrafish results in a more limited or focal pattern of infection, with acid-fast bacilli aggregating along the lining of the swim bladder and pericardial cavity as well as in ovaries and eggs.29 Scrolls may prove more efficient for organisms that are scattered in a variety of tissues, whereas core samples may work best with organisms that typically form large aggregates or have a known affinity for or an increased prevalence in specific organs or tissues. The core technique offers 2 major advantages. First, it eliminates the risk of cross-contamination during sectioning of paraffin blocks and thus the need for negative controls between sampling of specimens.34 Second, zebrafish for histology are usually divided into 2 pieces by using a midsagittal cut after fixation; the pieces are embedded together and positioned so that sections are taken from this location. Acid-fast stained slides can therefore be matched so that cores can be obtained from precise locations in any organ where infected tissue is identified. Mycobacteria, including nonpathogenic strains, are common in freshwater aquaria.22,30,36 The intestinal lumen of zebrafish is often colonized by these bacteria; consequently, scrolls from a fish with extraintestinal mycobacteriosis likely could be contaminated by gut mycobacteria that are not involved with the disease.18
We also evaluated both the purity and concentration of DNA isolated from FFPE samples to determine whether these factors affected the ability of the assay to detect infection on the basis on sampling technique. However, neither DNA purity nor DNA concentration rendered one sampling technique superior over the other. Because of the relatively low diagnostic yield from FFPE samples, we recommend testing multiple (5 or 6) zebrafish for detection of infection in a population, in view of our current results. This strategy is practical because typically multiple zebrafish from a given population are submitted for histology for evaluation of sentinels or when an outbreak occurs. We are providing these tests to the zebrafish community currently, and they were recently used in pathogen survey studies at 2 large facilities.11,13
In conclusion, the simplex qPCR assays we developed are sensitive, specific, repeatable, and reproducible for the detection of the 3 most pertinent Mycobacterium pathogens of zebrafish. Future applications for these assays include screening for newly acquired fish to improve biosecurity and as a diagnostic test for laboratories with high rates of mortality or morbidity. To further minimize the resources needed for detecting infection, the development of a multiplex qPCR would be beneficial, because it is a more efficient and economical approach than are simplex assays.
Acknowledgments
We thank Andree A. Hunakapiller and Virginia G. Watral for technical assistance and the Sinnhuber Aquatic Research Laboratory at OSU for providing the zebrafish.
The work was supported by internal funds from the Veterinary Diagnostic Laboratory at OSU, the NIH NCRR T32 Training Grant on Aquatic Models RR023917 (DM), the NIH ORIP 2R24OD010998-13, and NIEHS Environmental Health Science Center #P30 ES000210.
References
- 1.Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. 2000. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp Med 50:666–672. [PubMed] [Google Scholar]
- 2.Carson FL. 1997. Histotechnology: a self-instructional text. 2nd ed. Chicago (IL): ASCP Press Chicago. [Google Scholar]
- 3.Eltoum I, Fredenburgh J, Myers RB, Grizzle WB. 2013. Introduction to the theory and practice of fixation of tissues. J Histotechnol 24:173–190. [Google Scholar]
- 4.Gray TJ, Kong F, Jelfs P, Sintchenko V, Chen SC. 2014. Improved identification of rapidly growing Mycobacteria by a 16S-23S internal transcribed spacer region PCR and capillary gel electrophoresis. PLoS One 9:e102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josephson KL, Gerba CP, Pepper IL. 1993. Polymerase chain reaction detection of nonviable bacterial pathogens. Appl Environ Microbiol 59:3513–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kembel SW, Wu M, Eisen JA, Green JL. 2012. Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance. PLoS Comput Biol 8:e1002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. 2004. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem and Physiol C Toxicol Pharmacol 138:383–390. [DOI] [PubMed] [Google Scholar]
- 8.Kent ML, Buchner C, Watral VG, Sanders JL, Ladu J, Peterson TS, Tanguay RL. 2011. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Organ 95:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent ML, Spitsbergen JM, Matthews JM, Fournie JW, Murray KN, Westerfield M. [Internet] 2012. Diseases of Zebrafish in Research facilities. ZIRC Health Services Zebrafish Disease Manual. [Cited 24 April 2012]. Available at: http://zebrafish.org/zirc/health/diseaseManual.php.
- 10.Kim H, Kim SH, Shim TS, Kim MN, Bai GH, Park YG, Lee SH, Chae GT, Cha CY, Kook YH, Kim BJ. 2005. Differentiation of Mycobacterium species by analysis of the heat-shock protein 65 gene (hsp65). Int J Syst Evol Microbiol 55:1649–1656. [DOI] [PubMed] [Google Scholar]
- 11.Mason T, Snell K, Mittge E, Melancon E, Montgomery R, McFadden M, Camoriano J, Kent ML, Whipps CM, Pierce J. 2016. Strategies to mitigate a Mycobacterium marinum outbreak in a zebrafish research facility. Zebrafish 13:S77–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray KN, Bauer J, Tallen A, Matthews JL, Westerfield M, Varga ZM. 2011. Characterization and management of asymptomatic Mycobacterium infections at the Zebrafish International Resource Center. J Am Assoc Lab Anim Sci 50:675–679. [PMC free article] [PubMed] [Google Scholar]
- 13.Murray KN, Varga ZM, Kent ML. 2016. Biosecurity and health monitoring at the Zebrafish International Resource Center. Zebrafish.13 Suppl 1:S30–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odumeru J, Gao A, Chen S, Raymond M, Mutharia L. 2001. Use of the Bead Beater for preparation of Mycobacterium paratuberculosis template DNA in milk. Can J Vet Res 65:201–205. [PMC free article] [PubMed] [Google Scholar]
- 15.Ostland VE, Watral VG, Whipps CM, Austin FW, St-Hilaire S, Westerman MF, Kent ML. 2007. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass (Morone chrysops x Morone saxatilis) and zebrafish (Danio rerio). Dis Aquat Organ 79:107–118. [DOI] [PubMed] [Google Scholar]
- 16.Peñuelas-Urquides Villarreal-Treviño L, Silva-Ramírez B, Rivadeneyra-Espinoza L, Said-Fernández S, León MB. 2013. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony-forming units. Braz J Microbiol 44:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson TS, Kent ML, Ferguson JA, Watral VG, Whipps CM. 2013. Comparison of fixatives and fixation time on PCR detection of Mycobacterium in zebrafish Danio rerio. Dis Aquat Organ 104:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson TS, Ferguson JA, Watral VG, Mutoji KN, Ennis DG, Kent ML. 2013. Paramecium caudatum enhances transmission and infectivity of Mycobacterium marinum and M. chelonae in zebrafish Danio rerio. Dis Aquat Organ 106:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poort MJ, Whipps CM, Watral VG, Font WF, Kent ML. 2006. Molecular characterization of a Mycobacterium species in nonnative poeciliids in Hawaii using DNA sequences. J Fish Dis 29:181–185. [DOI] [PubMed] [Google Scholar]
- 20.Pourahmad F, Thompson KD, Adams A, Richards RH. 2009. Detection and identification of aquatic mycobacteria in formalin-fixed, paraffin-embedded fish tissues. J Fish Dis 32:409–419. [DOI] [PubMed] [Google Scholar]
- 21.Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and biologist programmers. Methods Mol Biol 132:365–386. [DOI] [PubMed] [Google Scholar]
- 22.Rusin PA, Rose JB, Haas CN, Gerba CP. 1997. Risk assessment of opportunistic bacterial pathogens in drinking water. Rev Environ Contam Toxicol 152:57–83. [DOI] [PubMed] [Google Scholar]
- 23.Singh VM, Salunga RC, Huang VJ, Tran Y, Erlander M, Plumlee P, Peterson MR. 2013. Analysis of the effect of various decalcification agents on the quantity and quality of nucleic acid (DNA and RNA) recovered from bone biopsies. Ann Diagn Pathol 17:322–326. [DOI] [PubMed] [Google Scholar]
- 24.Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. 2006. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun 74:6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tacquet A, Tison F, Devulder B, Roos P. 1967. Functioning of the central laboratory for the identification of mycobacteria of the Pasteur Institute of Lille (France)—1961 to 1965. Bull Int Union Tuberc 39:28–35. [PubMed] [Google Scholar]
- 26.Watral V, Kent ML. 2007. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comp Biochem Physiol C Toxicol Pharmacol 145:55–60. [DOI] [PubMed] [Google Scholar]
- 27.Whipps CM, Watral VG, Kent ML. 2003. Characterization of a Mycobacterium sp. in rockfish Sebastes alutus (Gilbert) and Sebastes reedi (Westrheim and Tsuyuki) using rDNA sequences. J Fish Dis 26:241–245. [DOI] [PubMed] [Google Scholar]
- 28.Whipps CM, Dougan ST, Kent ML. 2007. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol Lett 270:21–26. [DOI] [PubMed] [Google Scholar]
- 29.Whipps CM, Matthews JL, Kent ML. 2008. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish (Danio rerio). Dis Aquat Organ 82:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whipps CM, Lieggi C, Wagner R. 2012. Mycobacteriosis in zebrafish colonies. ILAR J 53:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams SL, Harris NB, Barletta RG. 1999. Development of a firefly luciferase-based assay for determining antimicrobial susceptibility of Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol 37:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vadney FS, Hawkins JE. 1985. Evaluation of a simple method for growing Mycobacterium haemophilum. J Clin Microbiol 22:884–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ingen J. 2013. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med 34:103–109. [DOI] [PubMed] [Google Scholar]
- 34.Varanat M, Maggi RG, Linder KE, Horton S, Breitschwerdt EB. 2009. Cross-contamination in the molecular detection of Bartonella from paraffin-embedded tissues. Vet Pathol 46:940–944. [DOI] [PubMed] [Google Scholar]
- 35.Vetrovsky T, Baldrian P. 2013. The variability of the 16S rRNA gene in bacterial genomes and its consquenses for bacterial community analyses. PLoS One 8:e57923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanong RP, Pouder DB, Falkinham JO. 2010. Association of mycobacteria in recirculating aquaculture systems and mycobacterial disease in fish. J Aquat Anim Health 22:219–223. [DOI] [PubMed] [Google Scholar]
- 37.Zerihun MA, Hjortaas MJ, Falk K, Colquhoun DJ. 2011. Immunohistochemical and Taqman real-time PCR detection of mycobacterial infections in fish. J Fish Dis 34:235–246. [DOI] [PubMed] [Google Scholar]