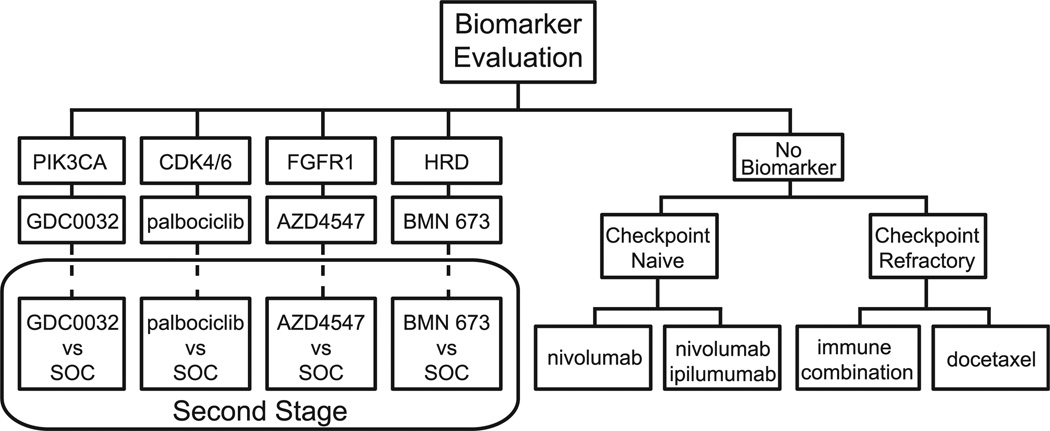
Figure 4.
S1400 (Lung-MAP). S1400 Uses An Umbrella Design to Investigate Second-Line Therapy in Treatment of Squamous-Cell Lung Cancer. Multiple Phase 2/3 Substudies With Rolling Opening and Closure Will Utilize Biomarker Profiling via Clinical Laboratory Improvement Amendments (CLIA)-Certified Next-Generation Sequencing Platforms. Patients Will Receive Matched Targeted Therapy Upon Identification of Genotypic Biomarker (eg, PIK3CA, CCND1, FGFR, or Homologous Repair Deficiency). If Threshold Response Rates are Surpassed, Targeted Drugs in Substudies Graduate to Randomized Comparison Versus Standard-of-Care Chemotherapy. Patients Without Actionable Biomarkers and Who Are Checkpoint Naive Will Be Randomized to The Anti–PDL-1 Agent Nivolumab or Combination Immunotherapy With Nivolumab–Ipilimumab. Patients Whose Disease Has Already Failed to Respond to Second-Line PD-1 Therapy (Checkpoint Refractory) Will Be Treated by A Third-Line Immunotherapy Combination Under Development