Table 1.
Reaction condition optimization.
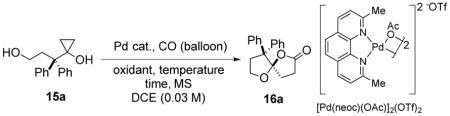
| ||||
|---|---|---|---|---|
| entry | Pd catalyst (equiv) | oxidant (equiv) | T (°C)/time (h) | yielda |
| 1 | Pd(OAc)2 (0.1) | BQ (2.0) | 60/14 | 13% |
| 2 | Pd(TFA)2 (0.1) | BQ (2.0) | RT/14 | 30% |
| 3 | Pd(TFA)2 (0.1)/PPh3 (0.2) | BQ (2.0) | RT/14 | LC |
| 4 | [(Cinnamyl)PdCl]2 (0.1) | BQ (2.0) | RT/60 | 66% |
| 5 | [Pd(neoc)(OAc)]2(OTf)2 (0.1) | BQ (2.0) | RT/60 | 85% |
| 6 | [Pd(neoc)(OAc)]2(OTf)2 (0.1) | O2 (balloon) | RT/60 | 60% |
| 7b | [Pd(neoc)(OAc)]2(OTf)2 (0.05) | BQ (2.0) | RT/60 | 57% |
| 8b | [Pd(neoc)(OAc)]2(OTf)2 (0.05) | BQ (2.0) | RT/60 | 77% |
| 9b | [Pd(neoc)(OAc)]2(OTf)2 (0.01) | BQ (2.0) | RT/60 | 63% |
| 10b | [Pd(neoc)(OAc)]2(OTf)2 (0.1) | BQ (2.0) | 40/36 | 79% |
| 11b | [Pd(neoc)(OAc)]2(OTf)2 (0.05) | BQ (2.0) | 50/18 | 89% |
| 12b | Pd(OAc)2 (0.1)/neocuproine (0.1) | BQ (2.0) | RT/60 | LC |
| 13b | Pd black recycled from reaction | BQ (2.0) | 50/18 | 0% |
Isolated reaction yields with 0.1 mmol of 15a;
Without MS; BQ: Benzoquinone; LC: Low Conversion; RT: Room Temperature; MS: 4Å Molecular Sieves.
