Abstract
Purpose
To investigate the anti-adhesive mechanisms of PXL01 in sodium hyaluronate (HA) by using the rabbit lactoferrin peptide, rabPXL01 in HA, in a rabbit model of healing tendons and tendon sheaths. The mechanism of action for PXL01 in HA is interesting since a recent clinical study of the human lactoferrin peptide PXL01 in HA administered around repaired tendons in the hand showed improved digit mobility.
Materials and methods
On days 1, 3, and 6 after tendon injury and surgical repair, reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) was used to assess mRNA expression levels for genes encoding the mucinous glycoprotein PRG4 (also called lubricin) and a subset of matrix proteins, cytokines, and growth factors involved in flexor tendon repair. RabPXL01 in HA was administered locally around the repaired tendons, and mRNA expression was compared with untreated repaired tendons and tendon sheaths.
Results
We observed, at all time points, increased expression of PRG4 mRNA in tendons treated with rabPXL01 in HA, but not in tendon sheaths. In addition, treatment with rabPXL01 in HA led to repression of the mRNA levels for the pro-inflammatory mediators interleukin (IL)-1β, IL-6, and IL-8 in tendon sheaths.
Conclusions
RabPXL01 in HA increased lubricin mRNA production while diminishing mRNA levels of inflammatory mediators, which in turn reduced the gliding resistance and inhibited the adhesion formation after flexor tendon repair.
Keywords: Adhesions, flexor tendons, lubricin, proteoglycan 4, PXL01
Introduction
PXL01 is a synthetic peptide derived from the human milk protein, lactoferrin. In vitro studies in human cell lines have shown that PXL01 exhibits an inhibitory effect on important hallmarks of adhesion formation by reducing secretion of inflammatory cytokines, promoting fibrinolysis, and reducing infections (1). Further, PXL01 in sodium hyaluronate (HA) reduced post-surgical adhesions in experimental models of abdominal surgery in rats (1) and flexor tendon repair surgery in rabbits (2,3). A recent clinical trial investigating the efficacy and safety of adjuvant treatment with PXL01 in HA after flexor tendon injury and repair in the hand showed increased digit mobility in PXL01 in HA-treated fingers compared to placebo (4). However, the molecular mechanisms underlying the effect of PXL01 in HA on adhesion formation and tendon healing have not been clarified. In this study, we used the corresponding peptide derived from rabbit lactoferrin (rabPXL01) to study the effects in a rabbit model of tendon surgery. We hypothesized that treatment with rabPXL01 in HA would lead to reduced inflammation and increased synthesis of proteoglycan 4 (PRG4), also called lubricin, after tendon injury and repair in rabbits. Increased lubricin levels and reduced inflammation could subsequently lead to inhibition of adhesion formation.
PRG4 is an interesting gene, and individuals carrying mutant variants of the gene can suffer a number of connective tissue complications (5). The protein it encodes is a mucinous glycoprotein secreted into synovial fluid by synovial fibroblasts (6) and the superficial zone cells of articular cartilage (7) and menisci (8). It has lubricating properties on articular cartilage (9), reduces synovial cell overgrowth, and protects the surface of the cartilage (10). Lubricin has also been found on the flexor tendon surface (11), and recent studies have shown that lubricin reduces tendon-gliding resistance (12,13). In the eye, the ocular surface epithelia express PRG4, which reduces friction between the cornea and conjunctiva (14). There is evidence that PRG4 may play a role in the biomechanics of rabbit ligaments during aging (15). PRG4 may also have anti-inflammatory effects itself (16). The expression of PRG4 is inhibited by tumor necrosis factor α (TNF-α) and interleukin (IL)-1, and stimulated by transforming growth factor β (TGF-β) (11). Thus, interfering with these inflammatory molecules may potentially increase lubricin levels after a tendon injury.
To investigate if the potential anti-adhesive effect of rabPXL01 in HA is mediated via PRG4, directly or indirectly by inflammatory mediators, and to further characterize the molecular effects of rabPXL01 in HA on tendon healing, we assessed mRNA levels for PRG4 and a subset of relevant molecules involved in the outcome early after rabbit flexor tendon repair (Table 1). The aim of this study is to provide potentially important information regarding the possible early molecular basis for the effects of rabPXL01 in HA on healing tendons and tendon sheaths.
Table 1.
Study data.
| Molecules studied | Clinical relevance |
|---|---|
| Proteoglycan 4 (PRG4)/lubricin | Lubrication factor |
| Collagen I, collagen III | Matrix molecules |
| Cyclo-oxygenase 2 (Cox-2) | Inflammatory mediator |
| Inducible nitric oxide synthase (iNOS) | Inflammatory mediator |
| Interleukin 1β, 6, 8 (IL-1β, IL-6, IL-8) | Inflammatory mediator |
| Tumor necrosis factor α (TNF-α) | Inflammatory mediator |
| Transforming growth factor β (TGF-β) | Critical growth factor |
| α Smooth muscle actin (αSMA) | Myofibroblast marker |
Materials and methods
Surgical procedure
Twenty-four female young adult New Zealand White rabbits weighing 3 kg (±0.3 kg) were divided into three groups (n = 8 for each group; killed 1, 3, or 6 days postoperatively). They were housed and cared for in accordance with regulations for the protection of laboratory animals. The local animal ethics committee approved the study (Permit Number: C 121/11). Rabbits were selected as experimental model since tendon repair surgery is feasible due to the sufficiently large size of the digit, while this type of surgery cannot be performed in mice or rats otherwise frequently used in non-clinical efficacy studies.
The surgical procedures were performed during anesthesia with fentanyl-fluanisone (0.3 mL/kg body weight; Hypnorm, Janssen, Beerse, Belgium) and midazolam (2 mg/kg body weight; Dormicum, Roche, Basel, Switzerland) under sterile conditions as described previously (17,18), and all efforts were made to minimize suffering. A prophylactic intravenous dose of the antibiotic cefuroxime (100 mg; Zinacef, GlaxoSmithKline, London, United Kingdom) was given before surgery. Buprenorfin (0.1 mg/kg body weight; Temgesic, RB Pharmaceuticals, Slough, United Kingdom) was given postoperatively to prevent pain. The surgical procedures were performed on both hind paws identically, as described previously (17,18). Briefly, to decrease the tensile load on the flexor tendon’s phalangeal section, a partial division of the tendons was performed at the tendon–muscle transition. The third digit was longitudinally incised, and the flexor tendon sheath opened approximately 10 mm between the first and second annular pulley. The superficial flexor tendon was resected, and the intermediate segment of the deep flexor tendon sharply divided transversely. The injured tendons were sutured end-to-end using a modified Kessler suture technique (5-0 Prolene, Ethicon, Sollentuna, Sweden) for the core suture, and then peripheral circumferential running sutures (6-0, PDS, Ethicon).
Administration of rabPXL01 in HA
RabPXL01 was prepared in HA as previously described (2), using a concentration of 20 mg/mL rabPXL01 in 1.5% HA (molecular weight 1.5–8.1 × 106 Da) (3). After surgery, the paws of the rabbits were randomized either to receive rabPXL01 in HA treatment or to be left untreated (paired control to account for any possible genetic influences on healing). RabPXL01 in HA was administered to the paw randomized to receive treatment (treatment paws were randomized for each animal and blinded to the surgeon up to the moment of product administration, and to all personnel involved in the evaluation) using a BD Neoflon 24GA catheter (Becton, Dickinson Infusion Therapy AB, Helsingborg, Sweden) connected to a 1 mL syringe containing the rabPXL01 in HA formula. The Neoflon catheter was inserted into the opening of the sheath and was still within the tendon sheath as the tendon sheath was closed with a running 6-0 PDS suture (Ethicon). When performing the final sutures, 0.3 mL of the rabPXL01 in HA formula was injected and the tendon sheath fully closed. The same procedure was performed in the untreated paw, but without injecting rabPXL01 in HA formula. The skin was closed with a running suture (5-0 Ethilon, Ethicon).
Sample collection
The tendons and sheaths were harvested 1, 3, or 6 days after surgery. The rabbits were sedated with midazolam (Dormicum, Roche) and euthanized by a lethal dose of Pentobarbitalnatrium (Apoteket, Uppsala, Sweden). Equally sized segments of tendons and sheaths, 4 mm proximal and 4 mm distal of the repair site, were harvested. The samples were cleaned from sutures and rinsed in physiological saline. The samples were put into tared cryovials, immediately placed in liquid nitrogen, and stored at –80 °C until further analysis.
RNA extraction and reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted from the tissue samples using the TRIspin method (19). The wet weight of the samples was assessed, and the frozen samples were pulverized using a Braun Micro-dismembrator (B. Braun Biotech International, Melsungen, Germany). After powdering, 1 mL of Trizol (Gibco Life Technologies, Carlsbad, CA) was added to each sample and the Trizol-powdered tissue was transferred to tubes and immediately put in liquid nitrogen until further analysis. The Trizol tubes were thawed at room temperature for subsequent analysis. After adding chloroform (0.3 mL; EMD Chemicals Inc., Gibbstown, NJ), the samples were centrifuged and the aqueous phase collected, to which ethanol was added to precipitate the RNA (70%, 0.6 mL). Using an RNeasy Total RNA Kit (Qiagen, Chatsworth, CA), RNA was eluted and quantified fluorometrically with Sybergreen (Mandel, Guelph, Ontario, Canada). RNA was further processed for reverse transcription to cDNA using a Qiagen Omniscript kit (Omniscript RT Kit, Qiagen, Mississauga, Ontario, Canada). cDNA was prepared for qPCR using IQ SYBR Green Supermix (Bio-Rad Laboratories Mississauga, Ontario, Canada). Primers previously utilized for rabbit tissues were added (Table 2) and the samples amplified using real-time PCR (iCycler iQ, Real Time PCR Detection System, Bio-Rad Laboratories). 18S was used as the house-keeping gene for normalization, and non-reverse transcribed RNA was used as a negative control to detect possible DNA contamination (none of the samples contained detectable DNA; data not shown).
Table 2.
Primer sequences.
| Gene | Primer sequence | Base pairs | Source | |
|---|---|---|---|---|
| 18S | Forward sequence | TGG TCG CTC GCT CCT CTC C | 360 | NR_003286 |
| Reverse sequence | CGC CTG CTG CCT TCC TTG G | |||
| αSMA | Forward sequence | GTG TGA GGA AGA GGA CAG CA | 446 | X60732 |
| Reverse sequence | TAC GTC CAG AGG CAT AGA GG | |||
| Collagen I | Forward sequence | GAT GCG TTC CAG TTC GAG TA | 312 | Personal communication |
| Reverse sequence | GGT CTT CCG GTG GTC TTG TA | |||
| Collagen III | Forward sequence | TTA TAA ACC AAC CTC TTC CT | 255 | Personal communication |
| Reverse sequence | TAT TAT AGC ACC ATT GAG AC | |||
| COX-2 | Forward sequence | CAA ACT GCT CCT GAA ACC CAC TC | 82 | NM_001082388 |
| Reverse sequence | GCT ATT GAC GAT GTT CCA GAC TCC | |||
| IL-1β | Forward sequence | GCC GAT GGT CCC AAT TAC AT | 121 | M26295 |
| Reverse sequence | ACA AGA CCT GCC GGA AGC T | |||
| IL-6 | Forward sequence | CCT GCC TGC TGA GAA TCA CTT | 51 | AF469048 |
| Reverse sequence | CGA GAT ACA TCC GGA ACT CCA T | |||
| IL-8 | Forward sequence | CAA CCT TCC TGC TGT CTC TG | 145 | NM_001082293 |
| Reverse sequence | GGT CCA CTC TCA ATC ACT CT | |||
| iNOS | Forward sequence | CTG TGA CGT CCA GCG CTA CA | 119 | AF469048 |
| Reverse sequence | GCA CGG CGA TGT TGA TCT CTC GCC CT | |||
| PRG4 | Forward sequence | GAA CGT GCT ATA GGA CCT TC | 287 | NM_00127709 |
| Reverse sequence | CAG ACT TTG GAT AAG GTC TGC C | |||
| TGF-β | Forward sequence | CGG CAG CTG TAC ATT GAC TT | 271 | AF000133 |
| Reverse sequence | AGC GCA CGA TCA TGT TGG AC | |||
| TNF-α | Forward sequence | TCT AGT CAA CCC TGT GGC CC | 51 | NM_00108 |
| Reverse sequence | GCC CGA GAA GCT GAT CTG AG |
Statistical analysis
Levels of mRNA for a subset of mediators involved in the healing of flexor tendons and tendon sheaths were assessed 1, 3, and 6 days postoperatively. Flexor tendons and tendon sheaths treated with rabPXL01 in HA were compared with its untreated paired control using repeated measures ANOVA. Table 3 is a supplement reporting the remaining data. All tests were two-sided, and P < .05 was considered statistically significant.
Table 3.
Influence of rabPXL01 in HA on mRNA expression of matrix proteins, growth factors, and inflammatory mediators by injured tendons and tendon sheaths.
| Percent of corresponding control (untreated tendons and tendon sheaths) |
P values |
||||||
|---|---|---|---|---|---|---|---|
| Molecule | Group | Day 1 | Day 3 | Day 6 | Day 1 | Day 3 | Day 6 |
| Collagen I | Tendon PXL01 | 390.2 | 144.2 | 112.3 | .13 | .387 | .742 |
| Sheath PXL01 | 60.1 | 87.2 | 42.2 | .494 | .563 | .082 | |
| Collagen III | Tendon PXL01 | 973.9 | 157.8 | 96.4 | .003 | .387 | .889 |
| Sheath PXL01 | 70.8 | 69.9 | 58.4 | .556 | .563 | .065 | |
| Cox-2 | Tendon PXL01 | 263.5 | 121.1 | 93.8 | .214 | .506 | .896 |
| Sheath PXL01 | 71.7 | 76 | 56.7 | .331 | .416 | .094 | |
| iNOS | Tendon PXL01 | 398 | 175.7 | 232.6 | .085 | .043 | .298 |
| Sheath PXL01 | 114.7 | 98.9 | 55.1 | .687 | .969 | .106 | |
| IL-1β | Tendon PXL01 | 153.6 | 110.3 | 82.7 | .194 | .782 | .581 |
| Sheath PXL01 | 64.6 | 95.7 | 51.7 | .432 | .876 | .04 | |
| IL-6 | Tendon PXL01 | 177.6 | 154.4 | 163.7 | .337 | .233 | .582 |
| Sheath PXL01 | 82.2 | 81.5 | 49 | .789 | .607 | .005 | |
| IL-8 | Tendon PXL01 | 192.9 | 110.7 | 88.4 | .093 | .79 | .744 |
| Sheath PXL01 | 39.8 | 106 | 53.6 | .265 | .824 | .009 | |
| TNF-α | Tendon PXL01 | 89.9 | 131.9 | 126.4 | .742 | .473 | .441 |
| Sheath PXL01 | 66.9 | 84.9 | 56.8 | .427 | .553 | .129 | |
| TGF-β | Tendon PXL01 | 189.8 | 112 | 92 | .256 | .724 | .554 |
| Sheath PXL01 | 45.4 | 74 | 48.6 | .409 | .304 | .106 | |
| αSMA | Tendon PXL01 | 333 | 133.2 | 154.4 | .367 | .602 | .447 |
| Sheath PXL01 | 43.1 | 63.2 | 56.9 | .304 | .161 | .139 | |
Levels of mRNA in tendons and tendon sheaths for each molecule are shown as percent of corresponding control (the tendon and tendon sheath of the untreated paw). P values are shown for each molecule on days 1, 3, and 6 after surgery.
Results
Rupture rate
Three tendon ruptures occurred after repair surgery (two ruptures in the same rabbit): two of the untreated tendons and one of the rabPXL01 in HA-treated tendons, giving a rupture rate of 8% for the untreated tendons and 4% for the rabPXL01 in HA-treated tendons.
The effect of rabPXL01 in HA on the expression of PRG4 mRNA in tendon and tendon sheaths
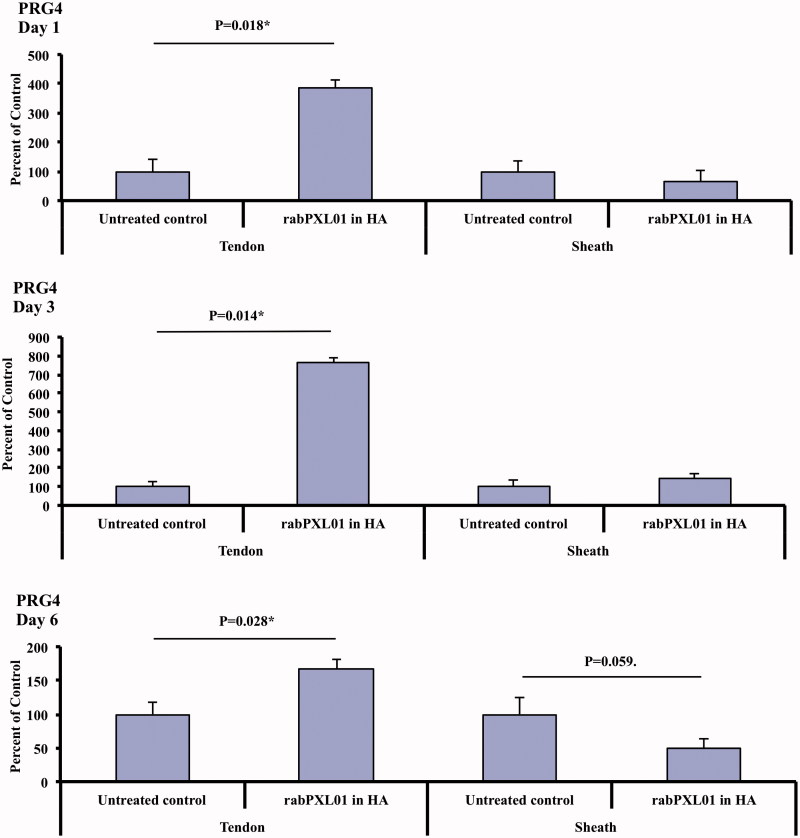
At all time points levels of PRG4 mRNA were higher in tendons treated with rabPXL01 in HA compared to untreated tendons. On the other hand, levels of PRG4 mRNA were similar in treated and untreated sheaths (Figure 1).
Figure 1.
Expression of PRG4 mRNA in flexor tendons and tendon sheaths treated with rabPXL01 in HA compared to untreated tendons and tendon sheaths 1, 3, and 6 days postoperatively. The following significance symbols are used: ***P < .001; **P < .01; *P < .05.
Influence of rabPXL01 in HA on mRNA levels for inflammatory mediators, transforming growth factor, the myofibroblast marker αSMA, and collagens I and III
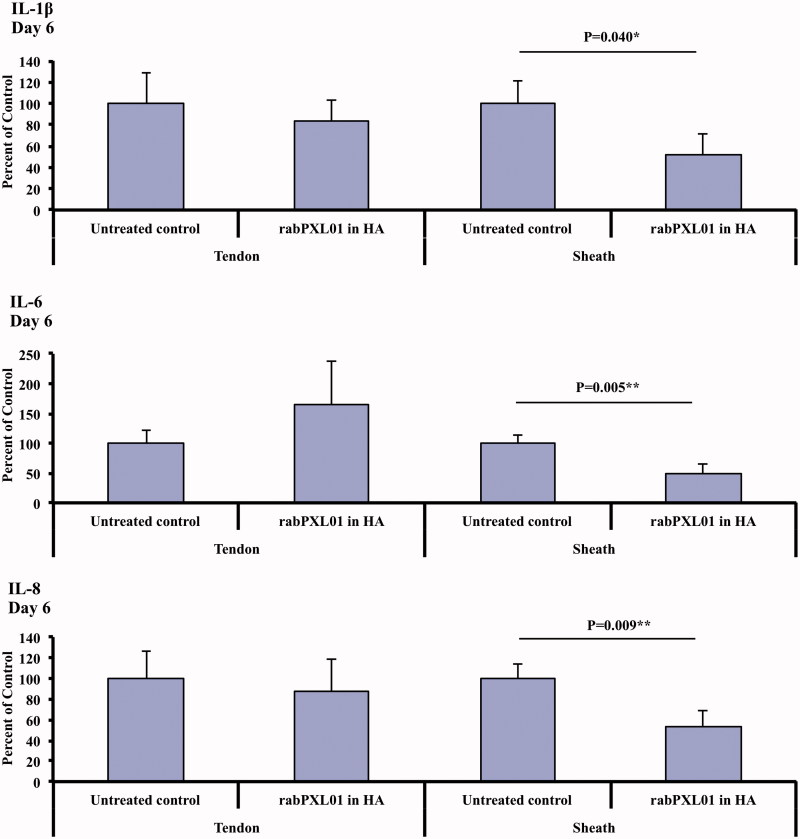
In tendon sheaths treated with rabPXL01 in HA, mRNA levels of IL-1β, IL-6, and IL-8 were lower than in untreated sheaths on day 6 (Figure 2, Table 3). Levels of mRNA for iNOS tended to be higher in rabPXL01 in HA-treated tendons than in untreated tendons at all time points, but with significant differences detected only on day 3 (P = .043, Table 3). The mRNA level of collagen III in rabPXL01 in HA-treated tendons was higher than that of untreated tendons on day 1 (P = .003). RabPXL01 in HA treatment did not significantly influence mRNA levels for any of the other molecules assessed, neither in tendons nor in tendon sheaths (Table 3).
Figure 2.
Expression of mRNA for IL-1β (top), IL-6 (middle), and IL-8 (bottom) in flexor tendons and tendon sheaths treated with rabPXL01 in HA compared to untreated tendons and tendon sheaths 6 days after surgery. The following significance symbols are used: ***P < .001; **P < .01; *P < .05.
Discussion
Previous studies on adjuvant treatment with PXL01 in HA have shown an increase in digit range of motion in both humans (4) and rabbits (2,3) without elevating the rate of rupture. The principal finding of the present study was an increased expression of PRG4 mRNA on days 1, 3, and 6 after surgery in tendons treated with rabPXL01 in HA. These findings suggest that rabPXL01 in HA treatment leads to an increase in lubricin production, and subsequently reduces the gliding resistance and inhibits adhesion formations after flexor tendon repair. The present findings are consistent with those reported by Hayashi et al. (12) who compared tendons in PRG4 knockout, heterozygous, and wild-type mice and showed that the gliding resistance of intrasynovial tendons from PRG4 knockout mice was higher than in the other groups. Adjuvant treatment with lubricin has also been evaluated in canine models of flexor tendon repair (13,20–22). Carbodiimide-derivatized HA and lubricin-treated tendons exhibited lower gliding resistance than tendons treated with HA alone (13). However, two of these studies that showed positive effects on digit function also reported adverse effects on tendon healing and decreased repair strength (21,22). These investigations suggested that carbodiimide-derivatized HA and lubricin may prevent cellular adhesions to tendon surfaces and enable lubrication of the tendon during flexion and extension, but this treatment also interferes negatively with tendon healing. In the present study we observed higher levels of PRG4 mRNA in rabPXL01 in HA-treated tendons, but no macroscopically obvious damage to the tendon tissue or altered rate of post-surgical tendon rupture. Previous studies using PXL01 in HA have shown no increase in the rate of tendon rupture and no tendon damages (2,3).
There were no detectable differences in mRNA levels in the tendons for the mediators IL-1, TNF-α, and TGF-β between treated and untreated groups. However, we found increased levels of PRG4 mRNA in rabPXL01 in HA-treated tendons but not in tendon sheaths. Receptors for PXL01 are not presently known, but likely the differences between PRG4 mRNA expression in tendons and tendon sheaths depend on PXL01 affinity for a tendon cell-specific receptor. Whether these cells are endogenous tendon cells or cells recruited to the injury site requires further investigation. These findings regarding PRG4 point to an interesting new target on which to focus future investigations, as well as to confirm that the mRNA changes observed are followed by increased protein production and secretion.
We have previously shown that PXL01 has anti-inflammatory effects on human macrophages by reducing the release of pro-inflammatory cytokines IL-1β, IL-6, and IL-8 (1). Consistent with these observations, the present study revealed a significant and co-ordinated suppression of mRNA levels for IL-1β, L-6, and IL-8 in tendon sheaths 6 days after surgery. Prolonged inflammation in the wound-healing cascade can lead to excessive adhesion formation, whereas down-regulation of the inflammatory response is believed to restrict proliferation and remodeling, leading to prevention of scarring (23). Suppression of inflammation may thus be one additional complimentary mechanism behind the improvement in digit mobility that has been shown after adjuvant treatment with PXL01 in HA (2–4).
The healing mechanisms in the tendon differ from the mechanisms operative in the tendon sheath. There is, for example, a change in collagen expression with increasing type III collagen in both the injured tendon and tendon sheath directly after an injury, whereas the level of collagen I remains unaltered in the tendon and increases in the tendon sheath at a later stage (17). This shift in collagen expression is believed to decrease tendon strength (24). In the present study, we observed higher levels of mRNA for both collagen I and collagen III in tendons treated with rabPXL01 in HA, at the earliest time point studied, day 1. An increase of both collagen I and collagen III production early in the healing process might create a stronger tendon tissue that can better withstand forces created during early active rehabilitation, with limited risk of repair rupture (24). Therefore, increased production of collagens I and III early after rabPXL01 in HA treatment might help to reduce the risk of repair rupture, but further studies are needed to support this notion.
HA as carrier allows for a slow release of rabPXL01 combined with initial lubricating properties around the tendon. HA has also shown some anti-inflammatory effects itself by reducing the concentration of inflammatory mediators (25). The current study design does not enable us to differentiate between the molecular effects of rabPXL01 versus HA, which is a limitation of the experiment. However, there were several reasons for the chosen design. Firstly, this study design mimics the design of the recently published randomized clinical trial where the treatment with a combination of PXL01 in HA improved hand function (4). Secondly, in the same rabbit model of tendon injury, adjuvant treatment with the combination of PXL01 in HA resulted in improved digit mobility compared to HA alone (3). Finally, using PXL01 alone is not technically feasible in this in vivo model, as the peptide does not remain at the wound site when applied in saline, but rather diffuses away rapidly.
In this study, tissue samples were harvested 1, 3, and 6 days after surgery. These time points were selected to address potential changes in the expression profile of different wound-healing mediators. It is known that molecular events such as those associated with an inflammatory response, which subsequently ‘set the stage’ for scar formation, take place shortly after the injury (26). Relevant to this point, Berglund et al. (18) detected temporal alterations in the pattern of mRNA expression for IL-1β and hyaluronan synthases, which reached peak levels 3 and 6 days after tendon repair, respectively. A limitation of the present study is that mRNA expression may not completely mirror the protein levels, since there may be post-transcriptional regulation of protein synthesis.
In summary, our results suggest that the anti-adhesive effect of PXL01 in HA involves an increased production of PRG4 in tendons and a decreased expression of inflammatory mediators in tendon sheaths.
Acknowledgements
The authors thank Britt-Marie Andersson for skillful laboratory assistance.
Disclosure statement
S.E. and M.W. have accepted consulting fees from Pergamum AB. C.R., D.A.H., and B.H. declare no conflicts of interest. M.M. is an employee of Pergamum AB. Pergamum AB was involved in designing the study and provided the rabPXL01 in HA formulation. However, Pergamum AB has not performed the surgery or expression analysis.
References
- 1.Nilsson E, Bjorn C, Sjostrand V, Lindgren K, Munnich M, Mattsby-Baltzer I, et al. . A novel polypeptide derived from human lactoferrin in sodium hyaluronate prevents postsurgical adhesion formation in the rat. Ann Surg. 2009;250:1021–8. [DOI] [PubMed] [Google Scholar]
- 2.Wiig M, Olmarker K, Hakansson J, Ekstrom L, Nilsson E, Mahlapuu M. A lactoferrin-derived peptide (PXL01) for the reduction of adhesion formation in flexor tendon surgery: an experimental study in rabbits. J Hand Surg Eur Vol. 2011;36:656–62. [DOI] [PubMed] [Google Scholar]
- 3.Hakansson J, Mahlapuu M, Ekstrom L, Olmarker K, Wiig M. Effect of lactoferrin peptide (PXL01) on rabbit digit mobility after flexor tendon repair. J Hand Surg Am. 2012;37:2519–25. [DOI] [PubMed] [Google Scholar]
- 4.Wiig ME, Dahlin LB, Friden J, Hagberg L, Larsen SE, Wiklund K, et al. . PXL01 in Sodium hyaluronate for improvement of hand recovery after flexor tendon repair surgery: randomized controlled trial. PLoS One. 2014;9:e110735. doi: 10.1371/journal.pone.0110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alazami AM, Al-Mayouf SM, Wyngaard CA, Meyer B. Novel PRG4 mutations underlie CACP in Saudi families. Hum Mutat. 2006;27:213. doi: 10.1002/humu.9399. [DOI] [PubMed] [Google Scholar]
- 6.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- 7.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–52. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher BL, Schmidt TA, Voegtline MS, Chen AC, Sah RL. Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J Orthop Res. 2005;23:562–8. [DOI] [PubMed] [Google Scholar]
- 9.Swann DA, Slayter HS, Silver FH. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem. 1981;256:5921–5. [PubMed] [Google Scholar]
- 10.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. . The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rees SG, Davies JR, Tudor D, Flannery CR, Hughes CE, Dent CM, et al. . Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593–602. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi M, Zhao C, Thoreson AR, Chikenji T, Jay GD, An KN, et al. . The effect of lubricin on the gliding resistance of mouse intrasynovial tendon. PLoS One. 2013;8:e83836. doi: 10.1371/journal.pone.0083836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, et al. . Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt TA, Sullivan DA, Knop E, Richards SM, Knop N, Liu S, et al. . Transcription, translation, and function of lubricin, a boundary lubricant, at the ocular surface. JAMA Ophthalmol. 2013;131:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton GM, Lemmex DB, Ono Y, Beach CJ, Reno CR, Hart DA, et al. . Aging affects mechanical properties and lubricin/PRG4 gene expression in normal ligaments. J Biomech. 2015;48:3306–11. [DOI] [PubMed] [Google Scholar]
- 16.Alquraini A, Garguilo S, D'Souza G, Zhang LX, Schmidt TA, Jay GD, et al. . The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353. doi: 10.1186/s13075-015-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berglund M, Reno C, Hart DA, Wiig M. Patterns of mRNA expression for matrix molecules and growth factors in flexor tendon injury: differences in the regulation between tendon and tendon sheath. J Hand Surg Am. 2006;31:1279–87. [DOI] [PubMed] [Google Scholar]
- 18.Berglund M, Hart DA, Wiig M. The inflammatory response and hyaluronan synthases in the rabbit flexor tendon and tendon sheath following injury. J Hand Surg Eur Vol. 2007;32:581–7. [DOI] [PubMed] [Google Scholar]
- 19.Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082–6. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Sun YL, Jay GD, Moran SL, An KN, Amadio PC. Surface modification counteracts adverse effects associated with immobilization after flexor tendon repair. J Orthop Res. 2012;30:1940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao C, Hashimoto T, Kirk RL, Thoreson AR, Jay GD, Moran SL, et al. . Resurfacing with chemically modified hyaluronic acid and lubricin for flexor tendon reconstruction. J Orthop Res. 2013;31:969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Sun YL, Kirk RL, Thoreson AR, Jay GD, Moran SL, et al. . Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2010;92:1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szpaderska AM, DiPietro LA. Inflammation in surgical wound healing: friend or foe? Surgery. 2005;137:571–3. [DOI] [PubMed] [Google Scholar]
- 24.Wang XT, Liu PY, Tang JB. Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. J Hand Surg Am. 2004;29:884–90. [DOI] [PubMed] [Google Scholar]
- 25.Mitsui Y, Gotoh M, Nakama K, Yamada T, Higuchi F, Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26:1032–7. [DOI] [PubMed] [Google Scholar]
- 26.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. [DOI] [PubMed] [Google Scholar]