Abstract
Stroke causes long-term disability, and rehabilitative training is commonly used to improve the consecutive functional recovery. Following brain damage, surviving neurons undergo morphological alterations to reconstruct the remaining neural network. In the motor system, such neural network remodeling is observed as a motor map reorganization. Because of its significant correlation with functional recovery, motor map reorganization has been regarded as a key phenomenon for functional recovery after stroke. Although the mechanism underlying motor map reorganization remains unclear, increasing evidence has shown a critical role for axonal remodeling in the corticospinal tract. In this study, we review previous studies investigating axonal remodeling in the corticospinal tract after stroke and discuss which mechanisms may underlie the stimulatory effect of rehabilitative training. Axonal remodeling in the corticospinal tract can be classified into three types based on the location and the original targets of corticospinal neurons, and it seems that all the surviving corticospinal neurons in both ipsilesional and contralesional hemisphere can participate in axonal remodeling and motor map reorganization. Through axonal remodeling, corticospinal neurons alter their output selectivity from a single to multiple areas to compensate for the lost function. The remodeling of the corticospinal axon is influenced by the extent of tissue destruction and promoted by various therapeutic interventions, including rehabilitative training. Although the precise molecular mechanism underlying rehabilitation-promoted axonal remodeling remains elusive, previous data suggest that rehabilitative training promotes axonal remodeling by upregulating growth-promoting and downregulating growth-inhibiting signals.
Keywords: stroke, rehabilitative training, axonal remodeling, corticospinal tract, motor map reorganization, motor system, neurotrophic factor, functional compensation, neural activity, growth promoting signal, growth inhibitory signal, task-specific training
Introduction
Stroke is one of the leading causes of death and disability worldwide, and the numbers of incident strokes, prevalent stroke survivors, disability-adjusted life-years lost owing to stroke, and stroke-related deaths are increasing (Feigin et al., 2014). Although thrombolytic treatments to restore blood flow during the acute phase of stroke were proven to be effective (Emberson et al., 2014), only a limited number of stroke patients can receive them because of a short therapeutic time window (Adeoye et al., 2011). Therefore, many stroke survivors experience persistent difficulty with daily tasks. Disability of upper extremities is a common impairment experienced by the majority of stroke survivors, and it strongly influences their quality of life. Although almost all stroke patients experience functional recovery to some extent, a complete recovery is rarely attained (Kwakkel et al., 2003). Rehabilitative therapy has been the most common treatment for chronic stroke patients. Task-specific training has been used for a long time, and it is considered as a more beneficial and reliable therapy than newly developed rehabilitative methods, such as transcranial magnetic stimulation, transcranial direct current stimulation, robotic therapy, or virtual reality in recent guideline for stroke rehabilitation (Winstein et al., 2016). However, despite the common use in clinical settings, the mechanism by which rehabilitative training promotes functional recovery remains to be elucidated.
After brain injury, various changes take place at cellular and network levels. Motor map reorganization is one of the phenomena induced by rehabilitative training after stroke. Rehabilitation-induced motor map reorganization was first demonstrated by Nudo et al. (1996) in his pioneering work. In this study, he discovered that the area that was originally non-hand area, such as shoulder area, turned into hand area if the animal was subjected to rehabilitative training of skilled hand movement after focal ischemic infarct over the hand area in the primary motor cortex. Motor map reorganization is believed to play critical roles in the recovery of motor function after stroke because several studies found a significant correlation between the extent of motor map reorganization and functional recovery (Traversa et al., 1997; Johansen-Berg et al., 2002). In addition to damaged primary motor cortex, motor map reorganization occurs in the secondary motor areas including the premotor area and the supplementary motor area, and the contralesional motor cortex. Although the mechanism by which the rehabilitative training induces motor map reorganization is not completely understood, animal studies during the last decade revealed that axonal remodeling or morphological alterations such as axonal extension and sprouting in the corticospinal tract play a pivotal role. While the relationship between motor map reorganization and axonal remodeling has been mainly investigated in the contralesional motor cortex, we recently reported that axonal remodeling in the corticospinal tract underlies rehabilitation-induced motor map reorganization in the ipsilesional secondary motor area (Okabe et al., 2016). In this review, we address the occurrence of axonal remodeling in the corticospinal tract of adult animals after stroke and the contributions of such events to motor map reorganization and functional recovery. Furthermore, we discuss the mechanism by which rehabilitative training promotes axonal remodeling.
Types of Axonal Remodeling in the Corticospinal Tract
To address the properties of axonal remodeling in the corticospinal tract, it is practical to consider several remodeling types that have been described in the literature. The type of axonal remodeling can be determined according to the location of the neurons that undergo axonal remodeling and the pattern of morphological changes in the axon. So far, three types of axonal remodeling have been reported. The first type is the recrossing of the corticospinal tract axons that were originally projecting to ipsilesional (unaffected) spinal cord from the contralesional motor cortex (Figure 1A). Most studies investigating axonal remodeling in the corticospinal tract after stroke focus on this type. Such axonal remodeling was reported during spontaneous recovery and was promoted by treatments such as anti-NogoA immunotherapy (Wahl et al., 2014) or chondroitinase ABC therapy (Soleman et al., 2012). In normal animals, electrical intracortical microstimulation (ICMS) to the forelimb area of the motor cortex elicits the movement of the forelimb contralateral to the stimulated hemisphere. In contrast, bilateral forelimb movements are elicited when ICMS is applied to the contralesional forelimb area of the motor cortex in animals affected by stroke that undergo axonal remodeling in the corticospinal tract. This result indicates that remodeled axon formed functional connections with denervated spinal cord. This was proven by Wahl et al. (2014) via pharmacogenetics. They demonstrated that selective silencing of corticospinal fibers projecting from the contralesional motor cortex to the denervated spinal cord diminishes the electromyography (EMG) responses of the affected forelimb after ICMS to the contralesional motor cortex. Furthermore, selective silencing of remodeled corticospinal fibers causes a reappearance of functional deficits indicating that this newly formed network contributes to the functional recovery. The second type of axonal remodeling is the collateral fiber formation from the corticospinal tract originally projecting from the surviving ipsilesional motor cortex to the caudal part of the spinal cord (Figure 1B). Starkey et al. (2012) reported that the corticospinal tract projecting from the ipsilesional hindlimb area to the lumbar spinal cord acquires new connections to the spinal segments that innervate the muscles in the forelimb. In this report, they showed a significant correlation between the amount of axonal projections from the hindlimb area in the motor cortex to the forelimb area of the spinal cord and functional recovery. Furthermore, they demonstrated the expansion of the area where electrical stimulation elicits both forelimb and hindlimb movements. The third type of axonal remodeling is the collateral fiber formation with axonal extension in the dorsal column in the corticospinal tract projecting from the surviving ipsilesional motor cortex to the rostral part of the spinal cord (Figure 1C). This type of axonal remodeling was reported in our recent publication (Okabe et al., 2016). We demonstrated that the neurons projecting from the ipsilesional rostral forelimb area (RFA) of the secondary motor cortex to the upper cervical cord that innervates the muscles in the trunk, neck, and shoulder, extended their axons in the dorsal columns and formed new connections with the lower cervical cord that innervates the muscles in the distal forelimb. Besides axonal remodeling, the size of RFA was significantly enlarged, which significantly correlated to the functional recovery. Because we observed a slight, although not statistically significant, increase in the number of neurons projecting to both the upper and lower cervical cord in the contralesional motor area as well, it seems that collateral fiber formation with axonal extension in the dorsal column may occur in the corticospinal tract projecting from the contralesional motor area. In addition to these three types of axonal remodeling, the existence of corticospinal neurons that have collateral branches to multiple spinal segments or ipsilateral projections should be noted. Some corticospinal neurons have been reported to have collateral branches extending to multiple spinal segments in intact animals that account for approximately 20–30% of neurons projecting to the cervical cord (Shinoda et al., 1979). Similarly, ipsilateral corticospinal projections also exist under normal circumstances. Although ICMS evoked contralateral body movement at the minimum threshold current, higher amplitudes of the current evoked bilateral body movement (Brus-Ramer et al., 2009). Therefore, it is very likely that the neurons with collateral branches to multiple spinal segments or ipsilateral projections strengthen the connections selectively to the denervated spinal segment and contribute to motor map reorganization and functional recovery.
Figure 1.
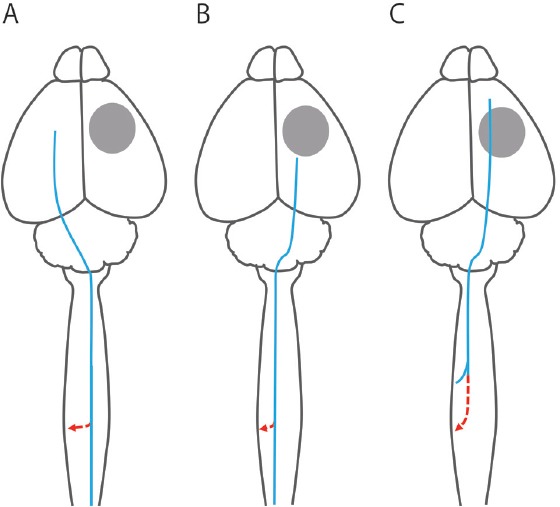
Type of axonal remodeling in the corticospinal tract.
The type of axonal remodeling in the corticospinal tract can be distinguished by comparing the original projection of the corticospinal axon and the newly formed one. The gray circle, the blue line, and the red line indicate the stroke lesion, original corticospinal axon, and newly formed corticospinal axon, respectively. The first type of axonal remodeling (A) is the recrossing of the corticospinal axon projecting from the contralesional hemisphere to the ipsilesional spinal cord which innervate forelimb or more caudal body part. The majority of previous studies investigating axonal remodeling after stroke have demonstrated this type of axonal remodeling. The second type of axonal remodeling (B) is the collateral formation from the corticospinal axon projecting from the ipsilesional hemisphere to the contralesional (affected) body part caudal to the area where a new axon is formed (e.g., hindlimb: Starkey et al., 2012). The third type of axonal remodeling (C) is the collateral formation accompanied by axonal extension in the dorsal column from the ipsilesional hemisphere to the contralesional body part rostral to the area where a new axon is formed (e.g., trunk: Okabe et al., 2016).
Considering the fact that the corticospinal tract can be remodeled regardless of whether it is projecting from the contralesional or ipsilesional hemisphere or whether it was originally projecting to the rostral or caudal part of the spinal segment to the area in which the new collateral is formed, it seems that all corticospinal axons can take part in axonal remodeling and motor map reorganization.
In all three types of axonal remodeling, corticospinal neurons alter their output selectivity from a single to multiple areas. In the first type of axonal remodeling, corticospinal neurons originally innervating the ipsilesional body part acquire the output to the contralesional body part (Figure 1A). In the second and third types of axonal remodeling, corticospinal neurons innervating the lower body part (e.g., hindlimb) and the proximal body part (e.g., trunk or neck), respectively, acquire the output to the forelimb (Figure 1B, C). Although the projections to a part of the spinal cord are not solely somatotopic (e.g., rostral to enlargement segment could reflect a projection to propriospinal neurons), increased number of projections to the denervated spinal segment should contribute to output related to the affected body part. A similar phenomenon was also reported in the sensory system. Using in vivo single cell calcium imaging, Winship and Murphy (2008) examined which part of the body had to be stimulated to produce a response by the neurons in the sensory cortex after stroke. In intact mouse, most neurons in the forelimb area of the sensory cortex respond only to stimulation in the contralateral forelimb. However, one month after stroke, the number of neurons responding to multiple limbs increased at the border between the forelimb area and the hindlimb area. The authors supposed that hardwired cortical neurons first adopt wider functional roles as they develop strategies to compensate for the loss of specific sensory modalities after stroke. Perhaps the corticospinal neurons also compensate for the lost motor function by weakening their functional specificity. Importantly, acquisition of multiple output results in the increased number of corticospinal neurons projecting to the target spinal cord area. Therefore, if the newly formed axon can affect the excitability of the motor neuron in the spinal cord, this means that axonal remodeling causes motor map reorganization (Figure 2).
Figure 2.
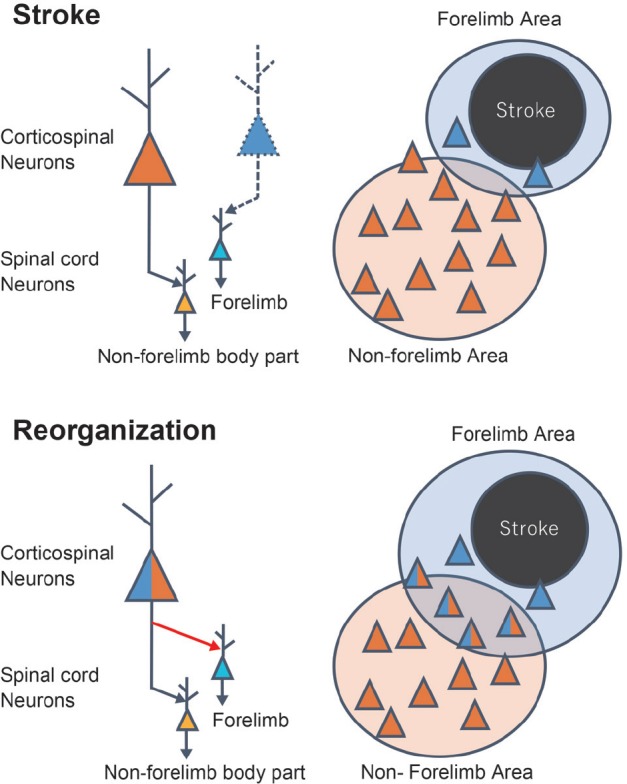
Mechanism of motor map reorganization by axonal remodeling in the corticospinal tract.
The scheme showing the presumed mechanism by which axonal remodeling induces motor map reorganization of the forelimb area. After stroke, a surviving corticospinal neuron projecting to the non-forelimb area in the spinal cord (orange neuron) remodels its axon to form a new connection (red arrow) with the neurons in the spinal area innervating the forelimb (light blue neuron). By this axonal remodeling, the number of neurons projecting to the both forelimb and non-forelimb area of the spinal cord (indicated by the blue/orange neuron) is increased (lower right panel). If these neurons can induce the depolarization of the motor neuron innervating the forelimb muscle, the forelimb area detected by intracortical microstimulation should expand.
It has not been very clear whether changes in the motor map lead to functional recovery or if the improved behavior leads to changes in the motor map. Nishibe et al. (2015) reported that rehabilitative training improved motor performance earlier than promoting reorganization of the motor map, which is consistent with our results (Okabe et al., 2016). This result suggests that the motor map changes do not lead to functional recovery. However, Nishibe et al. and we found that the responsiveness to electrical stimulation in preserved brain areas was dramatically impaired immediately after stroke because of diaschisis. Thus, this acute impairment may mask early motor map changes induced by rehabilitative training. Since both functional recovery and motor map reorganization are induced by various biological events such as axonal remodeling, dendritic arborization, and synaptogenesis in many types of neurons, including corticospinal neurons, spinal interneurons, and motor neuron, it is very hard to determine the relationship between them. Nevertheless, recent studies demonstrated a causal relationship between axonal remodeling and functional recovery (Ishida et al., 2016) or motor map reorganization (Wahl et al., 2014), suggesting that motor map reorganization and functional recovery do not share a cause-and-effect relationship, but both of them are commonly led by axonal remodeling.
Factors Influencing the Type of Axonal Remodeling in the Corticospinal Tract
We will now discuss which factors influence the type of axonal remodeling occurring in the corticospinal tract after stroke. One factor is the location of the area affected by stroke. As described above, the type of axonal remodeling depends on the original projection of the corticospinal neuron, or the body part originally controlled by the corticospinal neuron. Because the location of the corticospinal neuron is roughly determined by the body part innervated by the neuron as depicted in the motor homunculus, the type of axonal remodeling will depend on the area in which the surviving neuron is located. For example, while the number of corticospinal neurons projecting to the forelimb area of the spinal cord was increased in the ipsilesional hindlimb area in the experiment by Starkey et al. (2012), this number was not increased in our experiment because our stroke model destroyed more caudal areas including the hindlimb area (Okabe et al., 2016). Importantly, while the RFA and hindlimb areas were not completely destroyed in Starkey's study and our study, respectively, the number of corticospinal neurons projecting from these areas to the forelimb area of the spinal cord did not increase. Stroke causes prolonged neuronal dysfunction in peri-infarct areas (Brown et al., 2009). Therefore, it may be necessary that for axonal remodeling of corticospinal tracts that the surviving neurons preserve normal function.
In contrast, an increase of corticospinal projections in the contralesional hemisphere was commonly detected in many studies (Ueno et al., 2012; Bachmann et al., 2014). One reason may be that the contralesional hemisphere is never destroyed by stroke. It may also explain why the reports describing axonal remodeling in the corticospinal tract from the ipsilesional hemisphere are less numerous than those describing the remodeling in the contralesional hemisphere. It may be caused by the difficulty to induce a sufficiently large lesion that would also preserve an area to be analyzed in the ipsilesional hemisphere.
Another factor that influences the type of axonal remodeling in the corticospinal tract is the size of the area affected by stroke. In a previous study, Frost et al. (2003) found that, the larger the primary motor area lost after stroke in the monkey was, the larger was the premotor area gained in the monkey after recovery. Touvykine et al. (2015) performed a similar experiment in the rat. They induced a small or a large stroke in the caudal forelimb area (CFA) of the primary motor cortex and examined the size of RFA. They found a significant correlation between the lesion size and the size of the distal forelimb area in either contralesional or ipsilesional RFA. This result indicates that the area undergoing compensatory changes becomes broader as the lost function to be compensated for is greater. Because various compensatory alterations are observed in the peri-infarct area even when the stroke is very small (Brown et al., 2008, 2009), it is supposed that the surviving brain areas compensate for the lost function in order of a close relationship or a functional similarity to the destroyed brain area. For example, after stroke in the primary motor cortex, peri-infarct primary motor area compensates for the lost function first, and is then followed by the ipsilesional secondary motor area or the contralesional motor cortex (Touvykine et al., 2015). As mentioned above, the neurons that have similar projections to the destroyed neurons compensate for the lost function (e.g., neurons with branches to multi-segments or with ipsilateral projections). The surviving neurons may also remodel their axons in order of a morphological similarity of the axon to the destroyed neuron, because the neuron that has more similar projections needs less extensive axonal remodeling to compensate for the lost projections. Although the relationship between axonal remodeling and the lesion size has not been investigated in previous studies, considering the mutual relationship between them, it is very likely that the extent of the area undergoing axonal remodeling is also influenced by the lesion size. Interestingly, Touvikine et al. (2015) demonstrated that the size of the distal forelimb area in the contralesional or ipsilesional RFA correlated negatively with functional recovery, although this correlation was not statistically significant in the ipsilesional RFA. Similar results were also observed in the studies via functional magnetic resonance imagining (MRI) in human stroke patients, in which a negative correlation between excessive neural activity in the contralesional hemisphere and functional recovery was found (Ward et al., 2003; Calautti et al., 2007). It appears that these results are in conflict with the fact that the treatments promoting motor map reorganization improve functional recovery. One possible cause of this discrepancy is that each brain area has a limited compensatory capacity. As mentioned above, when larger neural tissue is destroyed by stroke, broader brain areas undergo compensatory alterations to satisfy increased functional demand. Thus, the total compensation undertaken by surviving brain areas seems to be greater if the lesion becomes larger (Figure 3A–a). Nevertheless, because each area has a limited compensatory capacity and the area contributing to the compensation becomes smaller when a larger part of the brain is destroyed by the lesion, the total compensation declines as the destruction of the brain becomes extensive (Figure 3A–b, -c). In addition, if the functional deficits caused by tissue destruction exceed the total compensation, a residual functional deficit will remain (Figure 3B). In this case, the surviving motor areas such as the contralesional motor cortex should compensate for the lost function with all their compensatory capacity, resulting in extensive plasticity changes detected by functional MRI or ICMS. Supporting this hypothesis, a disruption of neural activity in the contralesional motor area by transcranial magnetic stimulation causes a greater impairment in the patients with large stroke compared to the patients with a smaller one (Johansen-Berg et al., 2002), while the inhibition of the ipsilesional motor area is more disruptive in less affected patients (Friedman et al., 2004). Furthermore, it has been reported that the functional potential in chronic stroke patients depends on the structural integrity of the ipsilesional corticospinal tract (Stiner et al., 2007; Lindenberg et al., 2010), suggesting that the contralesional hemisphere may have a limited compensatory capacity compared to the ipsilesional hemisphere, at least in the case of human patients.
Figure 3.
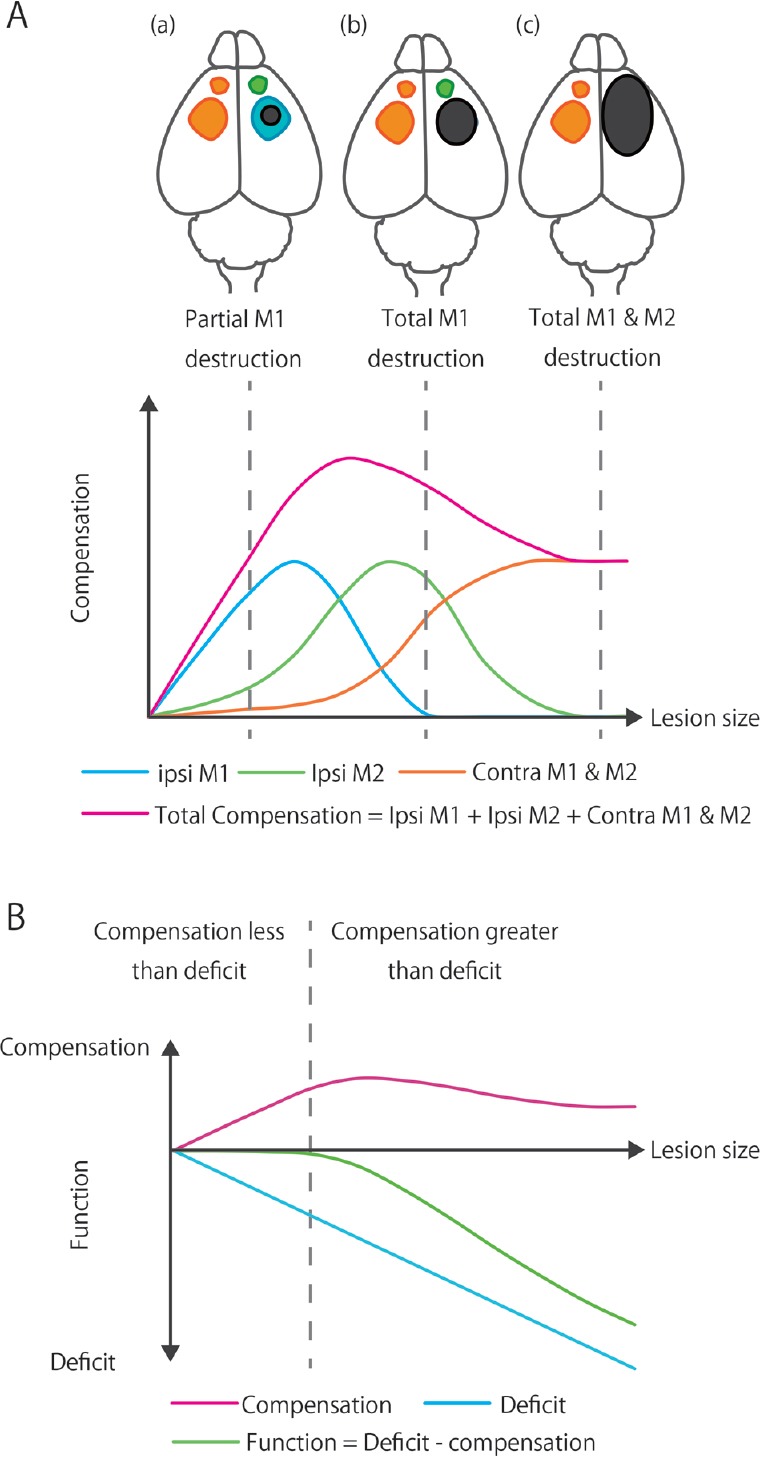
Relationship between functional compensation and lesion size.
(A) Images in the upper panel show the expansion of the brain lesion (Black area). Ipsilesional primary and secondary motor areas (ipsi M1 and ipsi M2: caudal and rostral forelimb area in rodents) are indicated as blue and green areas, respectively. The contralesional motor area (contra M1 and M2) is indicated as orange area. The graph in the lower panel indicates the relationship between the lesion size and functional compensation by each and total brain areas. The data from previous studies indicate that the broader the brain area that contributes to functional compensation is, the larger will be the brain area destroyed by the injury (Frost et al., 2003). When a small part of the primary motor cortex is destroyed, compensatory alterations are detected in the peri-infarct area (Brown et al., 2008, 2009). However, detectable alterations are not observed or are negligible in the ipsilesional secondary motor area or the contralesional motor area if the lesioned area is restricted (a) (Touvykine et al., 2015). As the lesion size becomes larger, the functional compensation by the surviving primary motor cortex becomes more significant to meet an increased demand (Touvykine et al., 2015). Because the expansion of the lesion causes a decrease in the area available for functional compensation, functional compensation diminishes with the increasing lesion size, and functional compensation by peri-infarct primary motor area becomes zero if the primary motor area is destroyed completely (b). As well as the primary motor area, compensation in the secondary motor area is also expected to increase with functional demand and decline with the loss of surviving tissue. Although contralesional hemisphere is not directly destroyed by stroke, it has a limited compensatory capability. Therefore, functional compensation by contralesional motor area reaches a plateau (c). Considering the relationship between the lesion size and functional compensation in each area, the total compensation is also presumed to increase with functional demand and decline with the loss of surviving tissue. (B) Image in the lower panel shows the relationship between the lesion size and compensation, initial deficit, or functional outcome. Basically, a larger lesion causes a more severe initial deficit, and functional compensation increases to meet the functional demand. However, since the compensatory capacity is limited as described in the panel (A), it is supposed that functional deficit exceeds the total compensation with the massive tissue damage. In that case, functional recovery is restricted and a certain degree of disability remains for a long time.
Axonal Remodeling Induced by Rehabilitative Training
Axonal remodeling can also be influenced by the therapeutic intervention after stroke. In previous studies, various therapeutics were reported to promote axonal remodeling and improve the functional outcome after brain injury. These include: blocking of axonal outgrowth inhibitors (e.g., anti-NogoA immunotherapy, Wahl et al., 2014; chondroitinase ABC, Soleman et al., 2012), administration of axonal outgrowth-promoting factors (e.g., neurotrophin, Duricki et al., 2016; basic fibroblast growth factor, Ramirez et al., 1999), cell transplantation (neural stem cells, Daadi et al., 2010; mesenchymal stem cells, Liu et al., 2008), brain stimulation (e.g., optogenetic stimulation, Cheng et al., 2014), and rehabilitation therapy. In the following paragraph, we will address the effect of rehabilitative training on axonal remodeling in the corticospinal tract and discuss potential mechanisms by which rehabilitative training promotes axonal remodeling. Although the number of studies investigating the effect of post-stroke training on axonal remodeling is not extensive, several of them reported axonal remodeling induced by rehabilitative training. These studies reported an increase in axonal remodeling following constraint induced movement therapy (CIMT; Maier et al., 2008; Zhao et al., 2013; Ishida et al., 2016), skilled reach training (Starkey et al., 2011; Lee et al., 2013; Okabe et al., 2016), and rotarod training (Nakagawa et al., 2013) using a rodent model of stroke. In many of these studies, axonal remodeling was investigated in the corticospinal tract originating from the contralesional hemisphere. However, recent studies revealed that rehabilitative training also promotes axonal remodeling in the corticospinal tract (Okabe et al., 2016) or corticorubral tract (Ishida et al., 2016) from the ipsilesional hemisphere. Bechmann et al. (2014) reported that axonal remodeling also occurs in the rubrospinal tract or the reticulospinal tract during spontaneous recovery after cortical stroke. These neural pathways projecting from deep brain areas to the spinal cord were believed to have a supportive role for the corticospinal tract. However, a recent study demonstrated an important role for these in fine motor control (Esposito et al., 2014). Whereas it remains elusive whether rehabilitative training influences remodeling of these pathways, deep brain areas such as red nucleus or reticular formation could be novel therapeutic targets if future studies reveal a contribution of these areas in the functional recovery after stroke.
The next question would be: how does rehabilitative training promote axonal remodeling? While rehabilitative training could promote axonal remodeling through glial interaction (Liu et al., 2014) or vascular remodeling (Muramatsu et al., 2012), it seems logical to assume that the most direct effect of rehabilitative training would be an increased activity in the neural circuit controlling the movement used for the training. So, how can increased neural activity promote axonal remodeling?
One possible mechanism is that the increased neural activity causes an increased production of trophic factors rendering the environment more suitable for axonal remodeling. Neuronal membrane depolarization leads to brain-derived neurotrophic factor (BDNF) expression through several mechanisms including calcium ion stimulated intracellular signaling (West et al., 2001) and DNA methylation (Martinowich et al., 2003). In addition, neural activity also increases the responsiveness to neurotrophic factors by increasing the density of trophic receptors on neuronal surface (Meyer-Frank et al., 1998; Goldberg et al., 2002). It is known that axonal outgrowth is promoted by trophic factors such as BDNF and ciliary neurotrophic factor (CNTF), and inhibited by inhibitory extracellular substrates such as myelin associated protein and chondroitin sulfate (Goldberg, 2003; Yiu and He, 2006). These extracellular environments change dynamically after stroke (Carmichael, 2006). Especially in the early period after stroke, neuronal plasticity is enhanced by the upregulation of growth-promoting genes and downregulation of growth-inhibiting genes (Carmichael et al., 2005). Ueno et al. (2012) reported that the remodeling of the corticospinal tract in the spontaneous recovery after stroke was prevented by the inhibition of BDNF expression in the spinal cord. This result indicates that axonal remodeling in the corticospinal tract is dependent on BDNF expression in the target area toward which the axons extend. Rehabilitative therapies, such as aerobic exercise (Himi et al., 2016) and CIMT (Ishida et al., 2015), were reported to increase BDNF expression in the brain. Although the effect of rehabilitative training on BDNF expression in the spinal cord remains unexplored, it is likely to be an increase in BDNF expression because running exercise was demonstrated to increase BDNF expression in the spinal cord of healthy rats (Gómez-Pinilla et al., 2002). In addition to the effects on growth-promoting signals, recent studies also demonstrated the effect of rehabilitative training on growth-inhibiting signals. Choi et al. (2016) demonstrated that task-specific training with skilled forelimb-reaching task normalized ephrin-A1 expression, which is upregulated in reactive astrocytes and inhibits axonal growth after stroke. Furthermore, it was also demonstrated that CIMT (Zhao et al., 2013) and running exercise (Li et al., 2015) decreased the expression of Nogo-A/Nogo-66 receptor 1 (NgR1)/Rho-A. Because myelin-associated protein Nogo-A inhibits axonal regeneration through its receptor NgR1 and the intracellular component Rho-A, downregulation of this pathway results in enhanced axonal regeneration. Rehabilitative training with skilled forelimb task promoted axonal remodeling specifically in the lower cervical cord that innervates the forelimb muscle, but not in the upper cervical cord that mainly innervates the muscles in the trunk and the neck in our study (Okabe et al., 2016). This result suggests that task-specific training such as skilled forelimb-reaching task can promote axonal remodeling specifically in the neural network necessary for the execution of the training. Considering the mechanism of rehabilitation-promoted axonal remodeling described above, task-specific training may selectively potentiate the neural connections by optimizing the extracellular environment through upregulating growth-promoting signals and downregulating growth-inhibiting signals in a region-specific manner.
Perspective
The knowledge of how axonal remodeling occurs after brain injury and how it can be promoted has been increasing in the recent years. However, previous studies were mostly focused on the strategies to promote axonal remodeling. Therefore, how remodeled axons form synapses and contribute to functional compensation was rarely investigated. In the study by Wahl et al. (2014), aberrant axonal termination and deleterious effect on the functional recovery were observed when anti-Nogo immunotherapy was performed in parallel with rehabilitative training, suggesting that proper synapse formation by the remodeled axon is essential for functional recovery. Considering the fact that rehabilitation is a process of re-learning motor skills, it is supposed that rehabilitative training also plays a significant role in the refinement of the neural circuit after the reconstruction through multiple output acquisition. Furthermore, the majority of previously performed studies have investigated axonal remodeling in the pathways projecting directly from the motor cortex or to the spinal cord. Nevertheless, mutual interactions between multiple brain areas such as the motor cortex, red nucleus, reticular formation, and the cerebellum are necessary for motor execution or learning (Battaglia-Mayer et al., 2013). A recent neuroimaging study revealed that the functional connectivity among the preserved brain areas predicted specific behavioral deficits, and the loss of interhemispheric communication across a set of regions was associated with impairment across multiple functions (Siegel et al., 2016). Thus, understanding how rehabilitative training influences the interactions between these brain areas may provide valuable insights for the therapeutic intervention. Currently, enormous efforts have been made to develop novel therapies to relieve chronic stroke patients of their difficulties in daily life. These therapies include: transcranial magnetic stimulation, transcranial direct current stimulation, stem cell therapy, and brain machine interface. They seem highly promising. However, since these therapies are basically premised to be used together with rehabilitative training, understanding the biological principles of rehabilitative training is essential to maximize their efficacy without inducing side effects.
Footnotes
Funding: This work was supported by the JSPSKAKENHI Grant-in-Aid for Scientific Research (B), Grant Numbers24700572 and 30614276.
Conflicts of interest: None declared.
References
- Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann LC, Lindau NT, Felder P, Schwab ME. Sprouting of brainstem-spinal tracts in response to unilateral motor cortex stroke in mice. J Neurosci. 2014;34:3378–3389. doi: 10.1523/JNEUROSCI.4384-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex. 2003;13:1009–1022. doi: 10.1093/cercor/13.10.1009. [DOI] [PubMed] [Google Scholar]
- Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29:1719–1734. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34:322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci U S A. 2014;111:12913–12918. doi: 10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DH, Ahn JH, Choi IA, Kim JH, Kim BR, Lee J. Effect of task-specific training on Eph/ephrin expression after stroke. BMB Rep. 2016;49:635–640. doi: 10.5483/BMBRep.2016.49.11.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Davis AS, Arac A, Li Z, Maag AL, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duricki DA, Hutson TH, Kathe C, Soleman S, Gonzalez-Carter D, Petruska JC, Shine HD, Chen Q, Wood TC, Bernanos M, Cash D, Williams SC, Gage FH, Moon LD. Delayed intramuscular human neurotrophin-3 improves recovery in adult and elderly rats after stroke. Brain. 2016;139:259–275. doi: 10.1093/brain/awv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Capelli P, Arber S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature. 2014;508:351–356. doi: 10.1038/nature13023. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, et al. (GBD 2010) and the GBD Stroke Experts Group (2014) Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Goldberg JL. How does an axon grow? Genes Dev. 2003;17:941–958. doi: 10.1101/gad.1062303. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Himi N, Takahashi H, Okabe N, Nakamura E, Shiromoto T, Narita K, Koga T, Miyamoto O. Exercise in the early stage after stroke enhances hippocampal brain-derived neurotrophic factor expression and memory function recovery. J Stroke Cerebrovasc Dis. 2016;25:2987–2994. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.017. [DOI] [PubMed] [Google Scholar]
- Ishida A, Isa K, Umeda T, Kobayashi K, Kobayashi K, Hida H, Isa T. Causal link between the cortico-rubral pathway and functional recovery through forced impaired limb use in rats with stroke. J Neurosci. 2016;36:455–467. doi: 10.1523/JNEUROSCI.2399-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Misumi S, Ueda Y, Shimizu Y, Cha-Gyun J, Tamakoshi K, Ishida K, Hida H. Early constraint-induced movement therapy promotes functional recovery and neuronal plasticity in a subcortical hemorrhage model rat. Behav Brain Res. 2015;284:158–166. doi: 10.1016/j.bbr.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim JH, Choi DH, Lee J. Effect of task-specific training on functional recovery and corticospinal tract plasticity after stroke. Restor Neurol Neurosci. 2013;31:773–785. doi: 10.3233/RNN-130336. [DOI] [PubMed] [Google Scholar]
- Li C, Wen H, Wang Q, Zhang C, Jiang L, Dou Z, Luo X, Zeng J. Exercise training inhibits the Nogo-A/NgR1/Rho-A signals in the cortical peri-infarct area in hypertensive stroke rats. Am J Phys Med Rehabil. 2015;94:1083–1094. doi: 10.1097/PHM.0000000000000339. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62:2022–2033. doi: 10.1002/glia.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu R, Takahashi C, Miyake S, Fujimura H, Mochizuki H, Yamashita T. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat Med. 2012;18:1658–1664. doi: 10.1038/nm.2943. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ueno M, Itokazu T, Yamashita T. Bilateral movement training promotes axonal remodeling of the corticospinal tract and recovery of motor function following traumatic brain injury in mice. Cell Death Dis. 2013;7:e534. doi: 10.1038/cddis.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe M, Urban ET, 3rd, Barbay S, Nudo RJ. Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model. Neurorehabil Neural Repair. 2015;29:472–482. doi: 10.1177/1545968314543499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Okabe N, Shiromoto T, Himi N, Lu F, Maruyama-Nakamura E, Narita K, Iwachidou N, Yagita Y, Miyamoto O. Neural network remodeling underlying motor map reorganization induced by rehabilitative training after ischemic stroke. Neuroscience. 2016;339:338–362. doi: 10.1016/j.neuroscience.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Ramirez JJ, Finklestein SP, Keller J, Abrams W, George MN, Parakh T. Basic fibroblast growth factor enhances axonal sprouting after cortical injury in rats. Neuroreport. 1999;10:1201–1204. doi: 10.1097/00001756-199904260-00008. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Zarzecki P, Asanuma H. Spinal branching of pyramidal tract neurons in the monkey. Exp Brain Res. 1979;34:59–72. doi: 10.1007/BF00238341. [DOI] [PubMed] [Google Scholar]
- Siegel JS, Ramsey LE, Snyder AZ, Metcalf NV, Chacko RV, Weinberger K, Baldassarre A, Hacker CD, Shulman GL, Corbetta M. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A. 2016;113:E4367–E4376. doi: 10.1073/pnas.1521083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleman S, Yip PK, Duricki DA, Moon LD. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain. 2012;135:1210–1223. doi: 10.1093/brain/aws027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey ML, Bleul C, Maier IC, Schwab ME. Rehabilitative training following unilateral pyramidotomy in adult rats improves forelimb function in a non-task-specific way. Exp Neurol. 2011;232:81–89. doi: 10.1016/j.expneurol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Bleul C, Zörner B, Lindau NT, Mueggler T, Rudin M, Schwab ME. Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain. 2012;135:3265–3281. doi: 10.1093/brain/aws270. [DOI] [PubMed] [Google Scholar]
- Touvykine B, Mansoori BK, Jean-Charles L, Deffeyes J, Quessy S, Dancause N. The effect of lesion size on the organization of the ipsilesional and contralesional motor cortex. Neurorehabil Neural Repair. 2015;30:280–292. doi: 10.1177/1545968315585356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Ueno M, Hayano Y, Nakagawa H, Yamashita T. Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain. 2012;135:1253–1267. doi: 10.1093/brain/aws053. [DOI] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schröter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–1255. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. In vivo calcium imaging reveals functional rewiring of single somatosensory neurons after stroke. J Neurosci. 2008;28:6892–6606. doi: 10.1523/JNEUROSCI.0622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhao M, Xiao T, Jolkkonen J, Zhao C. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke. 2013;44:1698–1705. doi: 10.1161/STROKEAHA.111.000361. [DOI] [PubMed] [Google Scholar]


