Abstract
Anthrax, caused by the environmental bacterium Bacillus anthracis, is an important zoonosis nearly worldwide. In Central Asia, anthrax represents a major veterinary and public health concern. In the Republic of Kyrgyzstan, ongoing anthrax outbreaks have been reported in humans associated with handling infected livestock and contaminated animal by-products such as meat or hides. The current anthrax situation has prompted calls for improved insights into the epidemiology, ecology, and spatial distribution of the disease in Kyrgyzstan to better inform control and surveillance. Disease control for both humans and livestock relies on annual livestock vaccination ahead of outbreaks. Toward this, we used a historic database of livestock anthrax reported from 1932 to 2006 mapped at high resolution to develop an ecological niche model–based prediction of B. anthracis across Kyrgyzstan and identified spatial clusters of livestock anthrax using a cluster morphology statistic. We also defined the seasonality of outbreaks in livestock. Cattle were the most frequently reported across the time period, with the greatest number of cases in late summer months. Our niche models defined four areas as suitable to support pathogen persistence, the plateaus near Talas and Bishkek, the valleys of western Kyrgyzstan along the Fergana Valley, and the low-lying areas along the shore of Lake Isyk-Kul. These areas should be considered “at risk” for livestock anthrax and subsequent human cases. Areas defined by the niche models can be used to prioritize anthrax surveillance and inform efforts to target livestock vaccination campaigns.
Introduction
Anthrax, caused by the spore-forming, environmental bacterium Bacillus anthracis, is a zoonotic disease that was recently posited as “undervalued” relative to its health and economic impacts on livestock, wildlife, and humans.1 Control of anthrax in humans can be achieved by limiting the disease in livestock through vaccination and proper outbreak management.2 However, in resource-limited areas, widespread vaccination may be financially prohibitive and untenable. Of particular concern are agrarian and resource-limited countries in the Caucasus2–4 and Central Asia,5–7 where independence from the former Soviet Union has hindered public health management because of decreased funding.5
In Central Asia, anthrax represents a major veterinary and public health concern5,7,8. In the Republic of Kyrgyzstan, ongoing anthrax outbreaks have been reported in humans associated with handling infected livestock and contaminated animal by-products such as meat or hides. A review of ProMed-mail reports from 1990 to 2013 suggests a high human incidence during livestock epizootics (www.promedmail.org). As one example, during a single outbreak in July 2009, over 167 persons were under observation for suspect anthrax from contaminated meat. The current anthrax situation has prompted calls for improved insights into the epidemiology, ecology, and spatial distribution of the disease in Kyrgyzstan to better inform control and surveillance.
Ecological niche modeling is one tool for evaluating a species' potential distribution for management or disease risk mapping.9 Ecological niche models (ENM) use pattern matching algorithms or statistical approaches to search for nonrandom relationships between occurrence locations (e.g., latitude/longitude pairs) and environmental data (such as climatic variables) to predict the potential geographic distribution of a species.9 Such models provide testable hypotheses of a species' distribution potential.10 ENM approaches have been used to predict the distribution of B. anthracis across several landscapes under current9,11–13 and future climatic conditions.9,14 Such models provide a “first estimate” of where the pathogen may persist and Kracalik and others7 suggested national passive surveillance should include the appropriate diagnostic tools and regional veterinary training to properly detect outbreaks in areas predicted by ENMs.
In this study, we developed a geographic database of livestock anthrax spanning several decades to describe its occurrence in Kyrgyzstan. We used a presence-only ecological niche modeling approach to estimate the potential geographic distribution of B. anthracis across Kyrgyzstan. The objective of these experiments was to better define the ecology of B. anthracis and identify areas where improved livestock control and surveillance could be prioritized within the national infectious disease monitoring priorities for Kyrgyzstan.
Methods
Anthrax occurrence data.
We constructed a geographic information system (GIS) of livestock anthrax using historical data from the Kyrgyz Institute of Biotechnology (KIBT) in Bishkek (Figure 1 ). Historical records from 1932 to 2006 were cataloged in the Nidus database; a self-contained data entry, editing, and review system maintained by KIBT built on the Microsoft Access (Redmond, WA) platform for veterinary epidemiologists. Nidus contains information on the date, livestock species, and number of individual animals infected (often recording mortality and survival status) for each outbreak. However, total livestock population on affected properties was rarely reported. For this study, an outbreak was defined as any location with one or more anthrax cases.
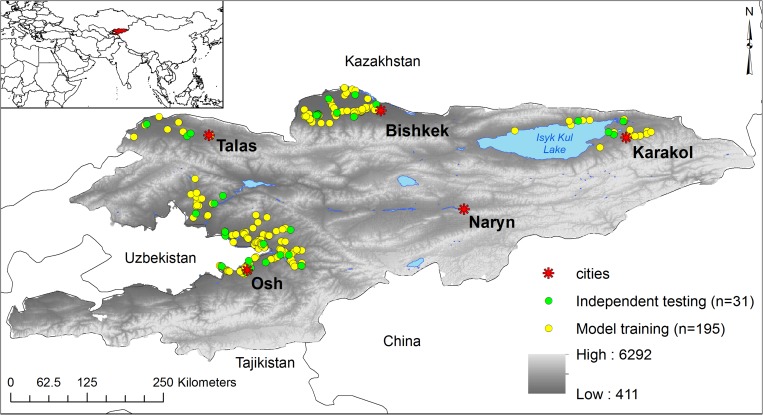
Figure 1.
Spatial setting of Kyrgyzstan in Central Asia (inset) and the spatial distribution of anthrax outbreak data to model the ecological distribution of Bacillus anthracis. All dots reflect the distribution of outbreaks in the country. Yellow dots represent the outbreak locations used to train the highest ranking model experiment in the study (experiment 8; Supplemental Table 1). Green dots represent data withheld to perform accuracy metrics on that experiment. Red stars represent major cities as landmarks to define anthrax foci in this study. The grayscale color ramp represents altitude in meters above sea level with higher elevations indicated in lighter shades.
We linked Nidus events with geographic coordinates recorded by KIBT using geographic positioning system receivers. From 2008 to 2010, KBIT personnel traveled to historical field sites and mapped each outbreak to the nearest possible location (e.g., carcass burial site, pasture, field, and farm) based on historical information, expert opinion, or recent known carcass locations. Historically, anthrax control in the former Soviet Union included covering anthrax burial sites with a layer of concrete (Figure 2 ), making them easy to find where they still exist. Mapping and analysis were performed in ArcGIS v10 (ESRI, Redlands, CA).
Figure 2.
Examples of burial sites from field efforts to map the geographic distribution of anthrax outbreaks. Historically, anthrax outbreaks were controlled by burying carcasses and pouring concrete slabs over the burial site. This practice was applied in (A) open pastures and (B) backyard settings.
Ecological niche modeling.
Ecological niche modeling experiments were performed using Desktop GARP (DG) version 1.1.3 (http://www.nhm.ku.edu/desktopgarp/). GARP is a presence-only modeling algorithm that has been extensively tested.15 The modeling system has been defined in detail elsewhere.16 Briefly, GARP is an iterative algorithm that searches for nonrandom relationships between point occurrences and environmental data. GARP develops a series of if/then logic statements, called rules that use one of four types (range, negated range, atomic, or logistic regression) to describe presence or absence of the target species in ecological space. Rules are developed and tested internally using random draws of presence points from known occurrences and pseudo-absences (background). An internal statistical test (a χ2 test) is used to evaluate the quality of each rule at predicting presence or absence with the user's predefined proportion of input data. GARP can accept, modify, or delete rules using deletions, insertions, cross-overs, etc. to improve predictive accuracy in a genetic fashion. Once a rule set (50 rules per model) is developed, it is projected onto the geographic landscape to develop a presence/absence map describing the species' potential geographic distribution. For examples of the relationship between rule sets and geographic predictions, see Mullins and others.17,18
Environmental data.
Environmental variables used in this study followed Mullins and others17 from a study of neighboring Kazakhstan to allow for comparison. Five bioclimatic variables describing measures of temperature and precipitation were downloaded from the WorldClim online database (www.worlclim.org).19 WorldClim variables are interpolated monthly measurements recorded at stations located worldwide between 1961 and 2000. WorldClim produces 19 “bioclim” variable grids to describe annual trends, seasonality, and ecological parameters such as temperature of the coldest and warmest months. Two normalized difference vegetation index (NDVI) values were obtained from the Trypanosomiasis and Land Use in Africa research group (Oxford, United Kingdom).20 These variables were derived from a temporal Fourier-processed time series of advanced very high-resolution radiometer satellite data to produce measures of NDVI mean (average annual NDVI), amplitude (annual change in NDVI), and phase (seasonality of NDVI). We also used four variables describing soil pH, moisture, organic content, and calcium concentration. Soil variables were derived from the Harmonized World Soil Database and were available at 1 km2 resolution.21 All coverages were resampled to 1 km2 (0.01 decimal degrees) and clipped to the boundaries of Kyrgyzstan in ArcView 3.3 (Environmental Systems Research Institute, Redlands, CA). Variables are listed by name and source in Table 1.
Table 1.
Environmental covariates used to develop ecological niche models of Bacillus anthracis in Kyrgyzstan
| Environmental variable | Variable name | Data source | Reference |
|---|---|---|---|
| Altitude (m) | alt | WorldClim | 22 |
| Mean annual temperature (°C) | bio 1 | ||
| Annual temperature range (°C) | bio 7 | ||
| Annual precipitation (mm) | bio 12 | ||
| Precipitation of the wettest month (mm) | bio 13 | ||
| Precipitation of the driest month (mm) | bio 14 | ||
| Average base saturation (%) | kgbsavg | HWSD | 24 |
| Average calcium concentration | kgcaavg | ||
| Average soil pH | kgphavg | ||
| Average soil organic content | kgsoilocav | ||
| TFA mean NDVI | wd0114a0 | TALA | 23 |
| TFA NDVI annual amplitude | wd0114a1 |
HWSD = Harmonized World Soil Database; NDVI = normalized difference vegetation index; TALA = Trypanosomiasis and Land Use in Africa; TFA = temporal Fourier analysis.
Model building and evaluation.
GARP models were evaluated with post hoc accuracy tests using independent Kyrgyz outbreak data withheld from the modeling experiments. Because of the iterative nature of GARP, the rule-set approach does not arrive at a single solution.18 Because of this, model performance can be affected by variation in input data.22 We developed 10 separate GARP experiments to evaluate the effect of input variability on model output. We used a different random subset of training and testing data for each experiment. We randomly selected 10 independent evaluation datasets of 25% of the occurrence points (N = 31) to withhold from GARP experiment to calculate accuracy metrics18 because it is preferable to use an independent subset of data rather than resubstitution to assess model accuracy.15,18,23 The remaining 75% of the occurrence points (N = 195) were used for model building. DG internally partitions training/testing data for model building and testing, which were set at 75% and 25%. To maximize GARP performance, model runs were set to a maximum of 1,000 iterations or until convergence of 0.01. The best subset procedure was used to select the 20 best models under a 10% hard omission threshold and a 50% commission threshold15 for a final 10-model best subset for each GARP experiment. Each 10-model best subset was summated in ArcGIS (ESRI, Redlands, CA) using the raster calculator to create a composite prediction.
Predictive accuracy for each GARP experiment was assessed using the independent dataset (i.e., the 25% of occurrence points withheld from model building). We evaluated each best subset using the area under the curve (AUC) in a receiver operating characteristic (ROC)24 analysis of the 25% independent datasets.18 The AUC has been used extensively in species distribution modeling, and measures the ability of a model to discriminate between sites where a species is present, versus those where it is absent25; an AUC of 1 indicates a perfect model, whereas an AUC of 0.5 defines a model that predicts no better than random.24
Because AUC scores alone may not describe the accuracy of a model,26 we also calculated measures of omission (false negatives) and commission (false positives).18 Total and average omission were calculated from the 10 best models' subsets and the independent test data. Total omission was calculated as the total number of independent test points predicted absent by the summated grid of all 10 best models. Average omission was calculated as an average omission across each of the 10 best models. Total and average commission were also calculated. Total commission was calculated as the total number of pixels predicted present across all 10 models divided by the total number of pixels in the study area. Average commission was calculated as the average of the total number of cells predicted present divided by the total number of pixels in the study area on a model-by-model basis for each of the 10 best models in the subset.
For this study, we calculated accuracy metrics for each of the 10 randomly subset datasets and ranked experiments by AUC and total omission, selecting the experiment that balanced a high AUC and low omission. The best model was selected to describe the ecological niche characteristics and potential geographic distribution of B. anthracis across Kyrgyzstan.
Results
Outbreak data.
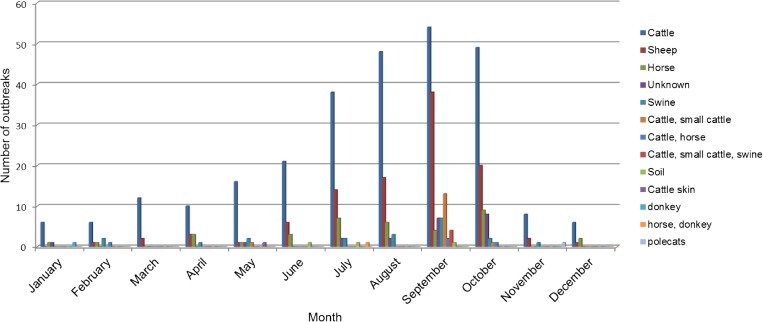
A total of 487 outbreaks from 1932 and 2006 were captured by the Nidus database (Table 2). Domestic cattle and sheep made up 56.3% and 21.6%, respectively, with horses, swine, unknown (unrecorded) species, donkeys, polecats (from fur farming operation6) and animal hides composing the remainder of the outbreaks.
Table 2.
Summary of anthrax outbreaks in Kyrgyzstan 1932–2006 by livestock group and proportion of the total
| Livestock species | Total outbreaks | % of outbreaks |
|---|---|---|
| Cattle | 274 | 56.3 |
| Sheep (small cattle*) | 105 | 21.6 |
| Horse | 37 | 7.6 |
| Unknown | 21 | 4.3 |
| Swine | 20 | 4.1 |
| Cattle, small cattle (mixed) | 15 | 3.1 |
| Cattle, horse (mixed) | 4 | 0.8 |
| Cattle, small cattle, swine | 4 | 0.8 |
| Soil | 3 | 0.6 |
| Cattle skin (animal hide) | 1 | 0.2 |
| Donkey | 1 | 0.2 |
| Horse, donkey | 1 | 0.2 |
| Polecats | 1 | 0.2 |
| Total | 487 | 100.0 |
Regionally, “small cattle” is used to describe mixed groups of sheep and goats.
Outbreaks per month are illustrated in Figure 3 . Outbreaks were reported in all months of the year, with cattle the only livestock group reported in every month. There was an increase in all groups in the late spring and summer months, with August–October having the highest numbers of outbreaks, as has been documented in other mid- to high-latitude regions.27,28
Figure 3.
Number of anthrax outbreaks reported by month by livestock group as defined by the national Kyrgyz Nidus anthrax database. Outbreaks were reported from 1932 to 2006 for this study.
Ecological niche modeling analysis.
The modeling process reached convergence of accuracy (0.01) prior to the maximum setting of 1,000 iterations in each experiment run for this study. All of the models had high-accuracy metric and broad agreement in the geographic prediction of B. anthracis, suggesting that the random selection of points had little influence on the rule sets developed. Experiment 8 had the highest AUC score of all 10 experiments run using random selections of testing data. Supplemental Table 1 summarizes the accuracy metrics and rank of all 10 experiments. The AUC score from the ROC analysis for experiment 8 was 0.9285 and was significantly different from a line of no information (P < 0.001). Average and total omission for experiment 8 were both 0%, meaning that all post hoc testing data were successfully predicted by all models in the best subset (Table 3). The predicted geographic distribution of B. anthracis across Kyrgyzstan is illustrated in Figure 4 as the summation of the 10-model best subset for experiment 8.
Table 3.
Sample sizes and accuracy metrics of the highest ranking ecological niche modeling experiment to predict the potential geographic distribution of Bacillus anthracis in Kyrgyzstan
| Metric | Model specifications |
|---|---|
| N to build models | 195* |
| N to test models (independent) | 31 |
| Total omission | 0 |
| Average omission | 0 |
| Total commission | 13.8 |
| Average commission | 21.12 |
| AUC | 0.9285† |
AUC = area under curve.
N was divided into 75% training/25% testing at each model iteration.
z = 11.61 (P < 0.001). Standard error = 0.0321.
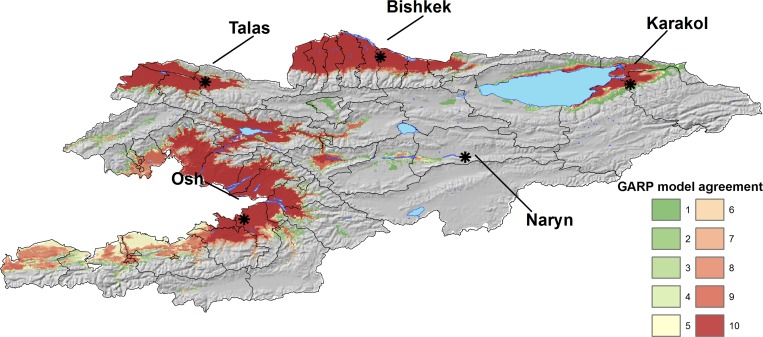
Figure 4.
Potential geographic distribution of Bacillus anthracis across Kyrgyzstan based on a GARP ecological niche modeling experiment. Darker red colors represent higher levels of individual model agreement in the best subset of 10 individual models within the highest ranking experiment. Areas with higher values can be interpreted as areas of greater likelihood of anthrax outbreaks in livestock. Gray hillshade reflects elevation across the landscape.
The predicted distribution of B. anthracis, as defined by areas of high model agreement in the best subset, was primarily restricted to four regions within Kyrgyzstan. The plateaus of northwestern and north central Kyrgyzstan, near Talas and Bishkek, respectively, were predicted with high model agreement as suitable areas for B. anthracis persistence. The north and western flatlands around Issyk-Kul Lake and the lowland areas of Osh near the Fergana Valley were also predicted with high model agreement. High mountain areas and high plateaus in the north were not predicted. Likewise, flatlands of south central Kyrgyzstan were also excluded. In addition, there was a patch of suitable habitat predicted with moderate model agreement west of Naryn in the central part of the country.
Discussion
We analyzed 74 years of livestock anthrax outbreaks using a combination of GIS and ecological niche modeling to provide insight into the ecology and geographic distribution of the disease in Kyrgyzstan. We had two main objectives in this study. First, we provided a historical analysis of livestock anthrax in Kyrgyzstan from 1936 to 2006 describing host species composition and outbreak seasonality. Second, we predicted the potential geographic distribution of B. anthracis for the country. Our study revealed discontinuous areas of ecological suitability for B. anthracis and localized hot spots of livestock anthrax transmission with four main regions of likely persistence: 1) the plateau of northwestern Kyrgyzstan near Talas, 2) the high plateau of north central Kyrgyzstan near the capital of Bishkek (Chuy oblast), 3) the steppe east of Issyk-Kul Lake (Issyk-Kul oblast), and 4) the low valleys of western Kyrgyzstan on the Uzbek border (Jalal-Abad, Osh, and Batken oblasts). These findings can be used to direct control efforts such as livestock vaccination.
Worldwide, domestic cattle are the most commonly reported livestock type with anthrax.5 Despite Kyrgyzstan maintaining a larger sheep population than cattle (National Statistical Committee of Kyrgyz Republic, stat.kg), we documented a larger proportion of outbreaks in cattle. In contrast, neighboring Kazakhstan reported more sheep outbreaks compared with cattle during a similar reporting period (1937–2005).6 Although differences may be due to variation in susceptibility or their geographic distribution, it has been suggested that as cattle are more valuable, sheep losses from anthrax may go undetected if outbreaks are small.5 Interestingly, sheep herding practices tend to include high-altitude summer pastures,29 which may reduce exposure to B. anthracis during summer months (based on our ENM predictions; Figure 4); though exposure to mid-elevation grasses could result in exposure during the migration up to or down from summer grazing.
As documented in previous studies,30,31 our findings indicated marked seasonality in anthrax reporting. Outbreaks increased from May to September and only cattle outbreaks were reported in every month. This late summer and early fall peak in anthrax has been reported at other mid- to high-latitude locations, including Texas.30 Vaccination campaigns should be targeted to livestock in mid to late spring ahead of the peak outbreak periods. This finding supports the hypothesis of nutritional stress and increased soil contact during drier times of year before moving to wintering pastures. Winter time outbreaks (at this latitude) have been previously attributed to food-borne contamination and moving stressed animals.31,32 Because of harsh winters in Kyrgyzstan, many animals are housed communally indoors during harsh winters and fed fodder (hay) prepared in the previous summer, raising the risk of food-borne outbreaks. Recent livestock anthrax outbreaks in Bangladesh were hypothesized to be food borne (fodder).33 Given that Kyrgyz livestock practices have raised concerns over adequate provision of winter nutrition,34 surveillance efforts should be aware of anthrax in all months.
The goal of an ENM experiment is to identify a combination of environmental or climatic variables that relate known occurrence points (here anthrax outbreaks) to regions on the landscape that can support the species of interest (here B. anthracis) beyond where sampling or reporting occurred. Research using GARP to model the distribution of B. anthracis in the United States and Kazakhstan has shown that the bacterium is likely limited by a combination of factors.11,14,17 In keeping with this research, we predicted four likely foci as suitable for B. anthracis. The steppe region of Talas reaches north and west into the Zhambyl area of Kazakhstan, previously defined as suitable B. anthracis habitat where hot spots of anthrax transmission occurred in cattle and small ruminants (sheep and goat) during a 40-year period.7 The little research on anthrax in Uzbekistan predicted the Fergana Valley, in the east, as suitable for B. anthracis.35 This valley leads into west central Kyrgyzstan, where we define the Osh anthrax focus (Osh, Jalal-Abad, and Batken oblasts). A review of archival ProMed records suggests a persistent anthrax problem (humans and animals) in Uzbekistan, similar to Kyrgyzstan, illustrating the need for joint surveillance and control in this transborder region. Although these areas of suitable B. anthracis habitat are relatively small, proper outbreak management and reporting of livestock mortality during seasonal animal migrations are needed to limit the spread of the disease.
The majority of GARP-defined B. anthracis habitat in Kyrgyzstan is grasslands (native grasses or cropland). Ecologically, these areas are similar to those long defined as habitat for large anthrax epizootics elsewhere.11 Pasture land increased in response to a livestock population crash fueled by the economic downturn following Soviet Union independence in the 1990s. Since then, Kyrgyz legislation and market demand have rebounded livestock populations to ∼1/2 of their pre-independence populations.36 This has been coupled with efforts by herders to settle pastures.37 From a practical standpoint, field epidemiologists and regional veterinarians can prioritize ecological zones for surveillance and control including seasonal migration routes. The majority of livestock is privately owned,34 so outreach should include efforts to engage household-level herders with educational materials on vaccination strategies and outbreak response.
Ecological niche modeling experiments have limitations that require discussion. Such approaches, by their definition and use of averaged climate data, may over generalize the landscape that supports viable populations of B. anthracis. Recent studies in Kazakhstan,17 the United States, and Italy12 have illustrated subtle, but likely important, differences in ENM predictions when models are restricted to single genetic lineages of B. anthracis (groups of genetically similar strains38). Comprehensive efforts have been completed to genotype B. anthracis in Kazakhstan,6 Italy,39 and the United States,40–42 such efforts have not yet been completed in Kyrgyzstan. Such studies should be a priority, as these data may aid in refining Kyrgyz models to specific genetic lineages. Aikembayev and others6 characterized genetic data from a limited number of Kyrgyz B. anthracis strains available in the Kazakh archive and identified at least two divergent lineages present in Kyrgyzstan, which may influence ENM predictions. At the same time, accuracy metrics for ENM predictions are still limited in value, in particular AUC.26 For this study, we also limited our inclusion of ecological variables to those used in recent studies of neighboring Kazakhstan to allow for comparison of a neighboring country with a well-studied anthrax situation. It is possible that alternative climatic variables, or combinations of variables, would refine the models built in this study. Future efforts can link the rule-set writing tools described in Mullins and others17 with additional variables and genetic data (as they become available) to refine the spatial patterns of this study.
In summary, the results of this study identify priority areas for anthrax surveillance, and anthrax livestock control based on the ENM predictions for Kyrgyzstan. Anthrax remains an important zoonosis in this country and across Central Asia, particularly in areas with limited public or veterinary health resources. The results of this study should inform anthrax policy in Kyrgyzstan and can be used to implement best practices for anthrax reporting and control in the areas most affected by the disease. Anthrax outbreaks concentrated, and suitable habitat predicted by the ENM experiments, along the Kazakh and Uzbek borders highlight the need for regional, transborder control efforts to ensure disease management in each of these countries. Recent foot and mouth disease outbreaks in Kazakhstan along these same border areas support our suggestion for multinational cooperation43 to control livestock diseases.
Supplementary Material
Footnotes
Authors' addresses: Jason K. Blackburn and Ian T. Kracalik, Spatial Epidemiology and Ecology Research Laboratory, Department of Geography, University of Florida, Gainesville, FL, and Emerging Pathogens Institute, University of Florida, Gainesville, FL, E-mails: jkblackburn@ufl.edu and itk@ufl.edu. Saitbek Matakarimov, Sabira Kozhokeeva, Zhyldyz Tagaeva, and Asankadyr Zhunushov, Kyrgyz Institute of Biotechnology, National Academy of Sciences, Bishkek, Kyrgyzstan, E-mails: acan@rambler.ru, ypmaboy@mail.ru, josmon@mail.ru, and junushov@mail.ru. Lindsay K. Bell, Spatial Epidemiology and Ecology Research Laboratory, Department of Geography, University of Florida, Gainesville, FL, E-mail: lindsaykbell@gmail.com.
References
- 1.Fasanella A, Galante D, Garofolo G, Jones MH. Anthrax undervalued zoonosis. Vet Microbiol. 2010;140:318–331. doi: 10.1016/j.vetmic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Kracalik I, Abdullayev R, Asadov K, Ismayilova R, Baghirova M, Ustun N, Shikhiyev M, Talibzade A, Blackburn JK. Changing patterns of human anthrax in Azerbaijan during the post-Soviet and preemptive livestock vaccination eras. PLoS Negl Trop Dis. 2014;8:e2985. doi: 10.1371/journal.pntd.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kracalik IT, Malania L, Tsertsvadze N, Manvelyan J, Bakanidze L, Imnadze P, Tsanava S, Blackburn JK. Evidence of local persistence of human anthrax in the country of Georgia associated with environmental and anthropogenic factors. PLoS Negl Trop Dis. 2013;7:e2388. doi: 10.1371/journal.pntd.0002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kracalik I, Malania L, Tsertsvadze N, Manvelyan J, Bakanidze L, Imnadze P, Tsanava S, Blackburn JK. Human cutaneous anthrax, Georgia 2010–2012. Emerg Infect Dis. 2014;20:261–264. doi: 10.3201/eid2002.130522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hugh-Jones M. 97 global anthrax report. J Appl Microbiol. 1999;87:189–191. doi: 10.1046/j.1365-2672.1999.00867.x. [DOI] [PubMed] [Google Scholar]
- 6.Aikembayev AM, Lukhnova L, Temiraliyeva G, Meka-Mechenko T, Pazylov Y, Zakaryan S, Denissov G, Easterday WR, Van Ert MN, Keim P, Francesconi SC, Blackburn JK, Hugh-Jones ME, Hadfield TL. Historical distribution and molecular diversity of Bacillus anthracis, Kazakhstan. Emerg Infect Dis. 2010;16:789–796. doi: 10.3201/eid1605.091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kracalik IT, Blackburn JK, Lukhnova L, Pazilov Y, Hugh-Jones ME, Aikimbayev A. Analysing the spatial patterns of livestock anthrax in Kazakhstan in relation to environmental factors: a comparison of local (Gi*) and morphology cluster statistics. Geospat Health. 2012;7:111–126. doi: 10.4081/gh.2012.110. [DOI] [PubMed] [Google Scholar]
- 8.Woods CW, Ospanov K, Myrzabekov A, Favorov M, Plikaytis B, Ashford DA. Risk factors for human anthrax among contacts of anthrax-infected livestock in Kazakhstan. Am J Trop Med Hyg. 2004;71:48–52. [PubMed] [Google Scholar]
- 9.Blackburn J. Integrating geographic information systems and ecological niche modeling into disease ecology: a case study of Bacillus anthracis in the United States and Mexico. In: O'Connell KP, Sulakvelidze EW, Bakanidze L, editors. Emerging and Endemic Pathogens: Advances in Surveillance, Detection, and Identification. Dordrecht: Springer; 2010. pp. 59–88. [Google Scholar]
- 10.McNyset KM. Ecological niche conservatism in North American freshwater fishes. Biol J Linn Soc Lond. 2009;96:282–295. [Google Scholar]
- 11.Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am J Trop Med Hyg. 2007;77:1103–1110. [PubMed] [Google Scholar]
- 12.Mullins JC, Garofolo G, Van Ert M, Fasanella A, Lukhnova L, Hugh-Jones ME, Blackburn JK. Ecological niche modeling of Bacillus anthracis on three continents: evidence for genetic-ecological divergence? PLoS One. 2013;8:e72451. doi: 10.1371/journal.pone.0072451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackburn JK, Odugbo MO, Van Ert M, O'Shea B, Mullins J, Perrenten V, Maho A, Hugh-Jones M, Hadfield T. Bacillus anthracis diversity and geographic potential across Nigeria, Cameroon and Chad: further support of a novel West African lineage. PLoS Negl Trop Dis. 2015;9:e0003931. doi: 10.1371/journal.pntd.0003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyner TA, Lukhnova L, Pazilov Y, Temiralyeva G, Hugh-Jones ME, Aikimbayev A, Blackburn JK. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PLoS One. 2010;5:e9596. doi: 10.1371/journal.pone.0009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epp T, Argue C, Waldner C, Berke O. Spatial analysis of an anthrax outbreak in Saskatchewan, 2006. Can Vet J. 2010;51:743–748. [PMC free article] [PubMed] [Google Scholar]
- 16.Kracalik I, Lukhnova L, Aikimbayev A, Pazilov Y, Temiralyeva G, Blackburn JK. Incorporating retrospective clustering into a prospective cusum methodology for anthrax: evaluating the effects of disease expectation. Spat Spatio-Temporal Epidemiol. 2011;2:11–21. doi: 10.1016/j.sste.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Bezymennyi M, Bagamian KH, Barro A, Skrypnyk A, Skrypnyk V, Blackburn JK. Spatio-temporal patterns of livestock anthrax in Ukraine during the past century (1913–2012) Appl Geogr. 2014;54:129–138. [Google Scholar]
- 18.Mullins J, Lukhnova L, Aikimbayev A, Pazilov Y, Van Ert M, Blackburn JK. Ecological niche modelling of the Bacillus anthracis A1: a sub-lineage in Kazakhstan. BMC Ecol . 2011;11:32. doi: 10.1186/1472-6785-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn JK, Hadfield TL, Curtis AJ, Hugh-Jones ME. Spatial and temporal patterns of anthrax in white-tailed deer, Odocoileus virginianus, and hematophagous flies in west Texas during the summertime anthrax risk period. Ann Assoc Am Geogr. 2014;104:939–958. [Google Scholar]
- 20.Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species' distributions: criteria for selecting optimal models. Ecol Model. 2003;162:211–232. [Google Scholar]
- 21.Stockwell D, Peters D. The GARP modelling system: problems and solutions to automated spatial prediction. Int J Geogr Inf Sci. 1999;13:143–158. [Google Scholar]
- 22.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 23.Hay SI, Tatem A, Graham A, Goetz S, Rogers D. Global environmental data for mapping infectious disease distribution. Adv Parasitol. 2006;62:37–77. doi: 10.1016/S0065-308X(05)62002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FAO/IIASA/CISS-CAS/JRC . Harmonized World Soil Database (Version 1.0) Rome, Italy and Laxenburg, Austria: FAO; 2008. [Google Scholar]
- 25.McNyset KM. Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecol Freshwat Fish. 2005;14:243–255. [Google Scholar]
- 26.Joyner TA. Ecological niche modeling of a zoonosis: a case study using anthrax and climate change in Kazakhstan, Masters Thesis. Department of Geography, University of Florida; Gainesville, Florida: 2010. p. 150. [Google Scholar]
- 27.Fielding A, Bell J. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24:38–49. [Google Scholar]
- 28.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 29.Wiley EO, McNyset KM, Peterson AT, Robins CR, Stewart AM. Niche modeling and geographic range predictions in the marine environment using a machine learning algorithm. Oceanography (Wash DC) 2003;16:120–126. [Google Scholar]
- 30.Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008;17:145–151. [Google Scholar]
- 31.Blackburn JK, Asher V, Stokke S, Hunter DL, Alexander KA. Dances with anthrax: wolves (Canis lupus) kill anthrax bacteremic plains bison (Bison bison bison) in southwestern Montana. J Wildl Dis. 2014;50:393–396. doi: 10.7589/2013-08-204. [DOI] [PubMed] [Google Scholar]
- 32.Crewett W. Introducing decentralized pasture governance in Kyrgyzstan: designing implementation rules. Environ Sci Policy. 2015;53:215–224. [Google Scholar]
- 33.Blackburn JK, Goodin DG. Differentiation of springtime vegetation indices associated with summer anthrax epizootics in west Texas, USA deer. J Wildl Dis. 2013;49:699–703. doi: 10.7589/2012-10-253. [DOI] [PubMed] [Google Scholar]
- 34.Turner A, Galvin J, Rubira R, Miller G. Anthrax explodes in an Australian summer. J Appl Microbiol. 1999;87:196–199. doi: 10.1046/j.1365-2672.1999.00869.x. [DOI] [PubMed] [Google Scholar]
- 35.Parkinson R, Rajic A, Jenson C. Investigation of an anthrax outbreak in Alberta in 1999 using a geographic information system. Can Vet J. 2003;44:315–318. [PMC free article] [PubMed] [Google Scholar]
- 36.Fasanella A, Garofolo G, Hossain M, Shamsuddin M, Blackburn J, Hugh-Jones M. Bangladesh anthrax outbreaks are probably caused by contaminated livestock feed. Epidemiol Infect. 2012;1:1–8. doi: 10.1017/S0950268812001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Gelder R. Livestock production and agriculture in Kyrgyzstan. Sci Access. 2004;1:200–203. [Google Scholar]
- 38.Van Ert M, Easterday W, Huynh L, Okinaka R, Hugh-Jones M, Ravel J, Zanecki S, Pearson T, Simonson T, Okinaka RT, Hugh-Jones ME, Ravel J, Zanecki SR, Pearson T, Simonson TS, U'Ren JM, Kachur SM, Leadem-Dougherty RR, Rhoton SD, Zinser G, Farlow J, Coker PR, Smith KL, Wang B, Kenefic LJ, Fraser-Liggett CM, Wagner DM, Keim P. Global genetic population structure of Bacillus anthracis. PLoS One. 2007;2:e461. doi: 10.1371/journal.pone.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fasanella A, Van Ert M, Altamura SA, Garofolo G, Buonavoglia C, Leori G, Huynh L, Zanecki S, Keim P. J Clin Microbiol. 2005;43:3398–3401. doi: 10.1128/JCM.43.7.3398-3401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackburn JK, Ten R, Aikembayev AM, Zhunushov A, Hugh-Jones MEM. Using Ecological Modeling with GIS to Improve International Anthrax Surveillance. New Orleans, Louisiana: Urisa GIS and Health Conference, May 2007; 2007. [Google Scholar]
- 41.Baibagushev E, Kreutzmann H, Abdulalishoev K, Zhaohui L, Richter J. Recent changes in pastoral systems. Case study on Kyrgyzstan. In: Kreutzmann H, Abdulalishoev K, Zhaohui L, Richter J, editors. Pastoralism and Rangeland Management in Mountain Areas in the Context of Climate and Global Change. Bonn, Germany: Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ); 2011. pp. 102–118. [Google Scholar]
- 42.Van Veen TWS. London, United Kingdom: Overseas Development Institute; 1995. The Kyrgyz Sheep Herders at a Crossroads. Pastoral Development Network Paper. [Google Scholar]
- 43.Sytnik I, Karibayev T, Tyulegenov S, Abenova A, Tashkenbayev A, Yerimbetov S. Surveillance of foot and mouth disease: a study of 2011–2012 outbreaks in Kazakhstan. Beтepинapнa Meдицинa. 2013;97:51–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.