Abstract
Purpose
To investigate the ratio of accommodative convergence per diopter of accommodative response (AC/A ratio) before, during, and after myopia onset.
Methods
Subjects were 698 children aged 6 to 14 years who became myopic and 430 emmetropic children participating in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error. Refractive error was measured using cycloplegic autorefraction, near work by parent survey, and the AC/A ratio by simultaneously monitoring convergence and accommodative response. The response AC/A ratios of children who became myopic were compared with age-, sex-, and ethnicity-matched model estimates for emmetropic children from 5 years before through 5 years after the onset of myopia.
Results
The response AC/A ratio was not significantly different between the two groups 5 years before onset, then increased monotonically in children who became myopic until reaching a plateau at myopia onset of about 7 Δ/D compared to about 4 Δ/D for children who remained emmetropic (differences between groups significant at P < 0.01 from 4 years before onset through 5 years after onset). A higher AC/A ratio was associated with greater accommodative lag but not with the rate of myopia progression regardless of the level of near work.
Conclusions
An increasing AC/A ratio is an early sign of becoming myopic, is related to greater accommodative lag, but does not affect the rate of myopia progression. The association with accommodative lag suggests that the AC/A ratio increase is from greater neural effort needed per diopter of accommodation rather than change in the accommodative convergence crosslink gain relationship.
Keywords: myopia, convergence, accommodation
The ratio of accommodative convergence that occurs per diopter (D) of accommodative response is referred to as the response AC/A ratio. Imbalances in this important cross-coupling gain relationship between these two key elements of clear and single binocular vision may produce clinically significant phoria or tropia.1 The AC/A ratio matures early in life and is stable across a broad span of ages. Values in infants 13 to 16 weeks of age were similar to those of pre-presbyopic adults, with adult-like ratios recorded in a subset of the most cooperative infants as early as 0 to 8 weeks of age.2 Cross-sectional studies report stable values in school-age children between 6 and 14 years3 and from infancy into adulthood for subjects as old as 46 years.4
Although the AC/A ratio matures early and changes little into adulthood, several factors may affect it: orthoptic training, presbyopia, cycloplegia, and refractive error. Of these, the neurologic or oculomotor changes that follow orthoptic training seem to have the least effect. Two weeks of orthoptic training increased vergence ranges and the degree of vergence adaptation to base-out prism, but the response AC/A ratio was virtually unchanged compared to baseline.5,6 Periods of orthoptic training longer than 2 weeks had no greater effect, producing either no significant change7 or small but temporary increases in the response AC/A ratio that dissipated within a year.8 However, the AC/A ratio has shown changes in animal and human studies during periods of vergence adaptation following short-term application of prisms and optical changes to the interpupillary distance.9–11
Older age has a much greater effect; the response AC/A ratio increases by roughly a factor of two as presbyopia approaches.12–14 This effect is presumably due to the increased effort needed to produce accommodative changes after age 30 years when accommodative amplitude begins its most rapid decline.15 Recent magnetic resonance imaging data show that adults in the age range of 30 to 50 years undergo the same ciliary muscle contraction per diopter of measurable accommodative response.16 The increase in the AC/A ratio suggests that more effort is needed to produce the same ciliary muscle contraction per diopter of accommodative response by the aging accommodative plant. Rather than poor muscle contractility, aging effects that might increase the effort needed per diopter of accommodation include increased tension on the ciliary muscle from choroidal sclerosis, documented in the rhesus monkey.17 The effort, or force of contraction of the ciliary muscle, needed per diopter of accommodation has been referred to as the “myopdioptre” by Fisher.18 This increase in the AC/A ratio with increased effort needed to accommodate is analogous to the two to three times increase in AC/A seen when cycloplegia impairs accommodation to 1 to 2 D of residual amplitude.19
The AC/A ratio also varies by refractive error, with higher AC/A ratios in myopic children compared to emmetropic children,3 a difference noted whether the AC/A ratio is assessed in childhood3,20 or in late-21 or early-onset myopic adults.22 An elevated AC/A ratio appeared at least 2 years before and 1 year after the onset of myopia23 and was a significant risk factor for myopia onset across baseline ages from 6 to 11 years.24 An increase in the AC/A ratio in myopic children could result from a higher crosslink gain relationship between accommodation and accommodative convergence. Another hypothesis is that the increased effort needed per diopter of accommodative output manifests as a higher AC/A ratio even though the accommodative convergence crosslink gain relationship may be relatively constant. Increased lag of accommodation, an obvious sign of impaired accommodation in presbyopia and cycloplegia, is well documented in myopic children compared to nonmyopic children.25,26
To our knowledge, there are no reports from other longitudinal studies in children on the AC/A ratio beyond these limited time points relative to the onset of myopia. The purpose of the current analysis is to extend the investigation of the temporal pattern for the response AC/A ratio in myopia development to time points beyond those previously reported, from 5 years prior through 5 years following myopia onset, in a large, ethnically diverse sample of children who became myopic compared to those who remained emmetropic. Additional analyses will explore the relationships between the response AC/A ratio and variables such as near phoria, accommodative lag, near work, and the rate of myopia progression in children once they become myopic.
Methods
Subjects were children participating between 1989 and 2010 in the Orinda Longitudinal Study of Myopia (OLSM), which expanded into the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study, a cohort study of ocular component development and risk factors for the onset of myopia in children of diverse ethnic backgrounds.27,28 Orinda Longitudinal Study of Myopia participants were from the predominantly white community of Orinda, California. To improve generalizability with respect to ethnicity, three additional clinic sites were added as part of CLEERE to recruit predominantly African American children (Eutaw, AL), Asian American children (Irvine, CA), and Hispanic children (Houston, TX). Testing of Native American children was conducted in Tucson, Arizona beginning in 2000 with the approval of the Tohono O'odham Nation Legislative Council. Supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, the study was conducted according to the tenets of the Declaration of Helsinki at the University of Alabama at Birmingham, the University of California, Berkeley, the University of Houston, The Ohio State University, the Southern California College of Optometry, and the University of Arizona. Each affiliated university's institutional review board approved informed consent documents. Parents provided written informed consent and children provided assent before the children were examined.
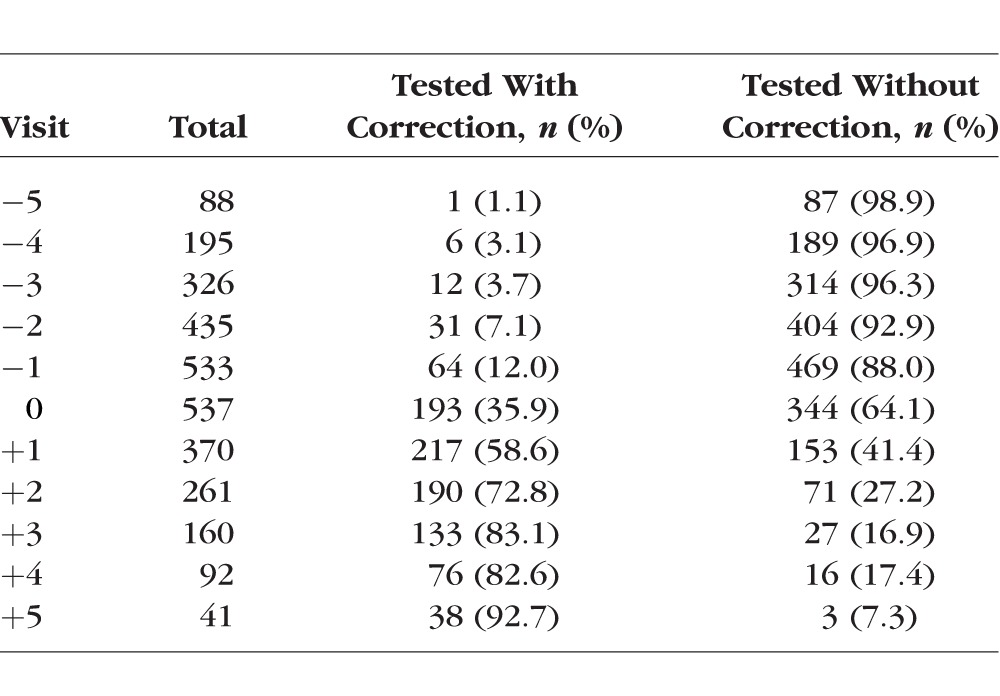
Ethnic group was determined by parental report on a medical history form using the six ethnic designations from the National Institutes of Health in 1997 when ethnic data were first gathered: American Indian or Alaskan Native; Asian or Pacific Islander; Black, not of Hispanic origin; Hispanic; White, not of Hispanic origin; Other, or Unknown. Ethnicity was assigned to the target ethnic group for the given site when parents provided more than one ethnic designation that included the site's targeted ethnicity. If parents provided more than one ethnic designation and neither included the site's targeted designation, ethnicity was assigned to the nonwhite of the two. Any missing parent-reported ethnicity was determined by investigator observation, a method that shows excellent agreement with parent-reported ethnicity.29 The sample for this report was 52% female with the following overall representation with respect to ethnicity: 14.5% Native American, 18.0% African-American, 18.3% Asian-American, 22.0% White, and 27.2% Hispanic. Children ranged in age from 6 to 14 years (first through eighth grade). Children classified as emmetropic were between −0.25 D and +1.00 D (exclusive of those endpoints) in each meridian at all study visits by cycloplegic autorefraction (n = 430). For a child to be included in the “became myopic” group, he or she must have had at least one nonmyopic visit followed by a visit where there was at least −0.75 D of myopia in each principal meridian on cycloplegic autorefraction. Children with at least −0.75 D of myopia in each principal meridian with no previous nonmyopic visits (i.e., prevalent myopes who had no identifiable onset visit) were excluded from the analysis (n = 367 of 904 myopes), leaving 537 children who became myopic for analysis. Table 1 gives the number of children who became myopic and had AC/A ratio data at each study visit. While the study began in 1989, AC/A ratio data were first collected in 1995. An additional 161 children became myopic after 1995 and provided AC/A ratio data included in the analysis of the relationship between the response AC/A ratio and the rate of myopia progression.
Table 1.
Number of Subjects Who Became Myopic With Both AC/A Ratio and Refractive Error Data by Visit and Refractive Error Correction Status During the Day of Testing
Trained and certified examiners measured the response AC/A ratio and the refractive error of children annually. The protocol for measurement of AC/A ratio has been described in detail elsewhere3 but is summarized here. Accommodative response was measured using the Canon R-1 auto-refractor (Canon, Lake Success, NY, USA; no longer manufactured) between 1989 and 2000 and the Grand Seiko WR 5100-K auto-refractor between 2001 and 2007 (Grand Seiko Co., Hiroshima, Japan). Both autorefractors were regularly calibrated against model eyes. The impact of the instrument change was less than 0.10 D.26 Children monocularly viewed a target consisting of a 4 × 4 grid of letters on a track behind a +6.50-D Badal lens positioned in front of the right eye, with each letter and space between letters subtending 38.75 minutes of arc at the eye (20/155 equivalent) at stimulus levels of 0 D, 2 D, and 4 D relative to infinity rather than to the far point of the subject. Subjects were instructed to keep the letters clear. Accommodative lag was similar using this Badal target compared to a physical near card at a 4-D stimulus level for emmetropic children and slightly higher for the Badal target for myopic children.26 We assume, however, that the AC/A ratio is independent of an individual subject's accommodative lag because the relationship between accommodation and accommodative convergence has been shown to be linear through a wide range of accommodative responses.30 Use of the Badal system has the added advantage of keeping size constant and limiting potential effects of proximity, particularly on vergence.31
Fusion was disrupted by wearing an infrared gel filter (Wratten 89B; Kodak, Rochester, NY, USA) over the left eye. The change in position of the left eye due to accommodative convergence was monitored with a second camera recording the relative position of the left eye's Purkinje images I and IV produced by an infrared light source atop the camera. Vergence was therefore asymmetric as the right eye remained in primary gaze throughout testing. Purkinje image movement in pixels was calibrated and converted into degrees of eye movement individually by having each child make a 10° lateral eye movement before testing. Children wore a refractive correction on the right eye during testing (subject's own contact lens or monocular trial lens) if they wore a correction to the testing session. The left eye under the infrared filter was always uncorrected to prevent unwanted reflections from glasses or contact lenses. Children who wore no correction to the testing session were left uncorrected. All accommodation and vergence data were recorded to tape simultaneously using a multiplexer for later image analysis. The AC/A ratio was calculated as the change in vergence in prism diopters per unit change in accommodative response between the 0-D and the 4-D stimulus levels (2-D data were not analyzed). AC/A ratio measurements at a visit were excluded if the accommodative response was less than 1.0 D, if the AC/A ratio was negative, or if the AC/A ratio was greater than 20 Δ/D under the assumption that the child either did not understand the task, was uncooperative, or had uncorrected myopia sufficient to neutralize the accommodative demand. Children were not excluded by these criteria, only particular study visits for a child.
Distance and near cover testing were performed by trained examiners while the child fixated a supra-threshold letter at distance and then at 40 cm, typical for clinical testing. Spectacle or contact lens corrections worn to the examination were worn during cover testing without adjustment to the prescription. If children were uncorrected and there was more than ±1.00 D of ametropia or 1.50 D or more of astigmatism, the refractive error correction from noncycloplegic retinoscopy was placed in a trial frame during cover testing. Children with less than that amount of ametropia who did not wear correction were left uncorrected during cover testing. The phoria measurement was the amount of prism required to neutralize movement during the alternating cover test.
Refractive error was tested after mydriasis and cycloplegia. When subjects had an iris color of grade 1 or 2,32 testing was done 30 minutes after one drop of proparacaine 0.5% and two drops of tropicamide 1%. When subjects had an iris color darker than grade 2, testing was done 30 minutes after one drop of proparacaine 0.5% and one drop each of tropicamide 1% and cyclopentolate 1%.33 For cycloplegic autorefraction, subjects fixated a reduced Snellen target through a +4.00-D Badal lens in primary gaze, allowing for children at any refractive error to have a target in focus for fixation without stimulating accommodation. The average of 10 autorefractor measurements were taken with the same autorefractor used for accommodative response.
Parents completed an annual survey form that asked the following question: “During the school year, how many hours per week (outside of regular school hours) would you estimate this child performs the following activities?” The activities listed were: “Studies or reads for school assignments; reads for fun (pleasure); watches television; uses a computer/plays video games; and engages in outdoor and/or sports activities.” Diopter-hours was defined as (3 × hours of reading + 3 × hours of studying + 2 × video/computer hours + hours of television watching).
Separate growth curves were constructed for emmetropic children for the response AC/A ratio as a function of age by sex and for each ethnic group. The data for emmetropic children can only be arranged by age because there is no corresponding “onset” point to arrange them by “visit” as with myopic children. The best-fitting models as defined by the Akaike Information Criterion were those that incorporated the natural log of age.34 These methods have been described in detail previously for modeling of emmetropic component development.35 The regression coefficients for ln(age) by sex and for each ethnic group were derived by mixed ANOVA modeling with repeated measures (SAS, version 9.3; SAS Institute, Inc., Cary, NC, USA).
The year the became-myopic subject first met the myopia criterion (at least −0.75 D of myopia in each principal meridian on cycloplegic autorefraction) was defined as year 0, the year of onset. The first study year before myopia onset was −1, 2 years prior was −2, and so forth out to −5 years before myopia onset. Each study year after onset for a given subject was designated +1, +2, and so forth out to +5 (Table 1). The age of each became-myopic subject at each study visit was applied to the appropriate emmetrope growth curve. This provided an age-, sex-, and ethnicity-matched emmetropic AC/A ratio for every became-myopic data point. Mixed modeling was then used to compare the mean difference between became-myopic data and the emmetrope model values as a function of study visit. A significance level of P < 0.01 was used in consideration of the large sample size. This level of adjustment is somewhat arbitrary but represents a compromise between filtering out spurious findings while allowing small differences to reach significance.
Results
The behavior of the response AC/A ratio before myopia onset, in the year of onset, and following onset is depicted in Figure 1. The AC/A ratio of emmetropic children was roughly 4.0 Δ/D with a small increase of about 10% over the 10-year span of visits. Values were not significantly different 5 years before myopia onset between children who remained emmetropic and children who became myopic. The response AC/A ratio then became significantly higher in children who became myopic compared to children who remained emmetropic 4 years prior to myopia onset and every year thereafter. The pattern of increase is noteworthy in that the AC/A ratio increased monotonically in each year before onset to reach 6.92 Δ/D at onset in year 0, a 1.6-fold increase compared to the value of 4.27 Δ/D in emmetropic children. Values after onset then plateaued, with no further significant changes compared to values at the onset of myopia. At year +5 (average age ± SD = 13.34 ± 0.74 years), there was no suggestion of a return toward normal, emmetropic values as has been reported for older teenage myopes.20
Figure 1.
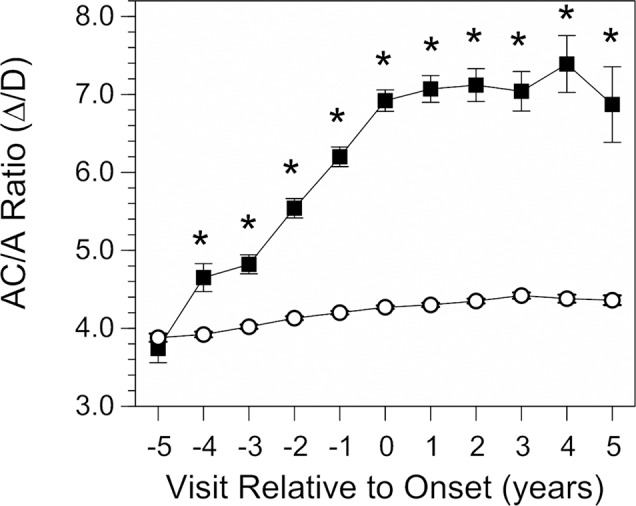
The response AC/A ratio as a function of annual visit relative to the onset of myopia (−5 years before to +5 years after onset, which is designated as visit 0). Data are from children who became myopic (▪) and from emmetrope model values (○). All error bars are ±SEM. Error bars for the emmetrope model values are smaller than the symbols. *Significant differences between groups (P < 0.01).
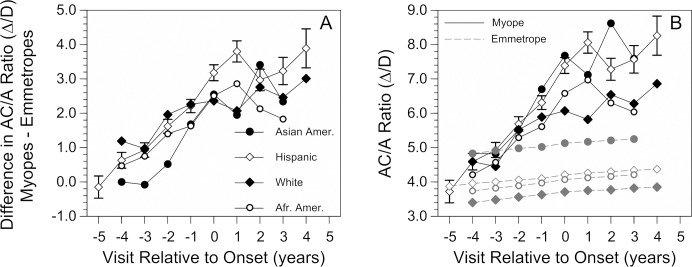
The general pattern of increasing AC/A ratio before myopia onset followed by a plateau at a higher level than emmetropic children was seen across all ethnic groups (Fig. 2A). The span of years over which the AC/A ratio was statistically significantly higher in children who became myopic compared to emmetropic children varied by ethnicity. It was longest in white children (9 years, −4 through +4) and Hispanic children (9 years, −3 through +5), followed by 8 years for African American children (−2 through +5), 6 years for Native American children (−3 through +2), and 5 years for Asian American children (−1 through +3). One exception to the general consistency of the results across ethnic groups was that Asian American emmetropic children had higher AC/A ratios compared to emmetropic children from other ethnic groups by approximately 1Δ/D (Fig. 2B).
Figure 2.
(A) The difference in response AC/A ratio between children who became myopic and age-, sex-, and ethnicity-matched emmetropic model values by visit relative to the onset of myopia. For example, Hispanic children at visit +3 years after myopia onset have a response ACA ratio that is approximately 3.2 Δ/D higher than their emmetropic model. Symbols for ethnic group are the same in the two panels. Instead of differences, panel (B) shows the average response AC/A ratio in children who became myopic (dark symbols and solid lines) and the corresponding age-, sex-, and ethnicity-matched emmetropic model values (gray symbols and dashed lines). Data are plotted where there were at least 20 children per visit for each ethnic group. For clarity, SE bars are shown for Hispanic children but were similar across ethnic groups.
The AC/A ratio was further analyzed with respect to near phoria, accommodative lag, and the rate of myopia progression. The increase in the response AC/A ratio prior to onset occurred at the same time that an increasing proportion of children had near esophoria ≥2Δ (Fig. 3). This percentage increased in monotonic fashion from 7.2% in year −5 to the highest proportion, 18.7%, in the year of myopia onset (P < 0.0001). Despite the stability of the elevated response AC/A ratio after onset, the percentage of myopic children with near esophoria then decreased in the years after onset to between 11% and 14% in years +3 through +5 (P = 0.008). In contrast, emmetropic children had a lower proportion of near esophoria that remained relatively stable across the range of ages in the study (3.8% at 6 years of age and 2.6% at 14 years of age). The presence of near esophoria ≥2Δ was more likely when the AC/A ratio was higher (odds ratio = 1.15, P < 0.0001) but was not significantly associated with accommodative lag in a univariate logistic regression (odds ratio = 0.90, P = 0.21). When the AC/A ratio and accommodative lag were adjusted for each other in a multivariate logistic model, the presence of near esophoria ≥2Δ was more likely when the AC/A ratio was higher (odds ratio = 1.17, P < 0.0001) and less likely with greater accommodative lag (odds ratio = 0.72, P = 0.001).
Figure 3.
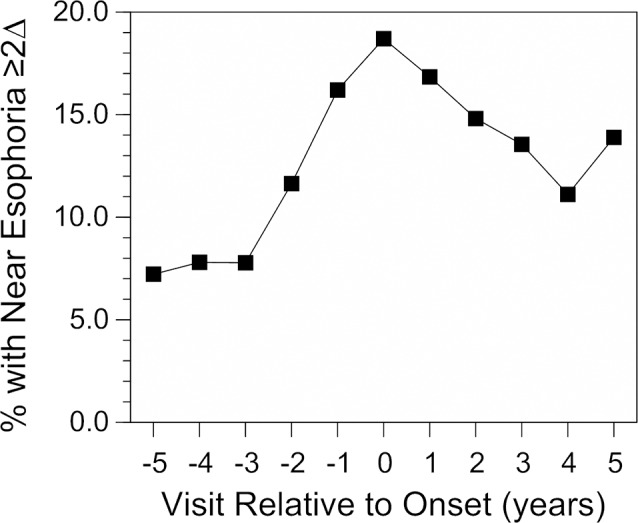
The proportion of children who became myopic with near esophoria ≥2 Δ/D by cover test as a function of visit relative to the onset of myopia. The proportion of emmetropic children with near esophoria ≥2 Δ/D was approximately 4% across the range of ages from 6 to 14 years.
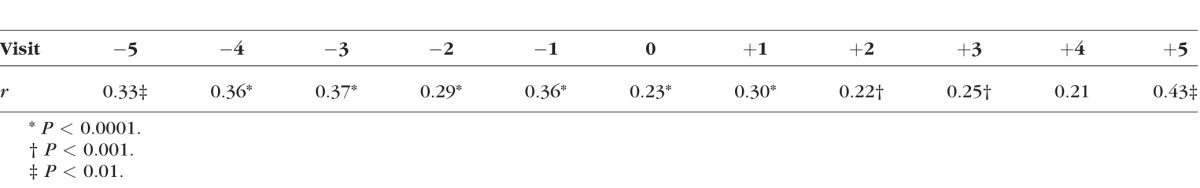
An elevated AC/A ratio might prompt a decrease in the accommodative response and an increase in accommodative lag in order to reduce asthenopia at near.20 Although the accommodative response data were monocular and therefore cannot address the implications of near esophoria or the AC/A ratio on binocular accommodative lag, there was no evidence of a substantial change across visits in the relationship between the AC/A ratio and monocular accommodative lag at the 4-D stimulus level (Table 2). The correlation was significantly positive at all visits except one (visit +4) and moderate in size (r = 0.22–0.43). These results suggest a consistent relationship across visits between greater lag and higher AC/A ratios despite the increase in AC/A ratio seen before myopia onset or the increase in accommodative lag seen after myopia onset.26
Table 2.
Correlation Coefficients for the Relationship Between the AC/A Ratio and Accommodative Lag at the 4-D Stimulus Level at Each Study Visit
There was no association between the response AC/A ratio and the rate of myopia progression (slope = −0.0010 D of progression per unit difference in Δ/D of AC/A ratio (95% CI = −0.011 to 0.009). This independence was consistent across different levels of near work (interaction P value for AC/A ratio by near work = 0.31). Although the AC/A ratio did not influence the rate of progression, children who became myopic and wore no correction for refractive error had a higher AC/A ratio than children who became myopic and wore a correction (P < 0.0001; Fig. 4). Refractive error correction was very uncommon at visit −2 (worn by 7% of children who eventually became myopic by our criterion) and became more common with time (36% of children wore a correction at the onset of myopia visit 0 and 73% wore a correction by visit +2). The difference in AC/A ratio between children wearing and not wearing a correction was on the order of 1.0 to 1.5 Δ/D and was statistically consistent across visits (difference by year interaction P value = 0.30).
Figure 4.
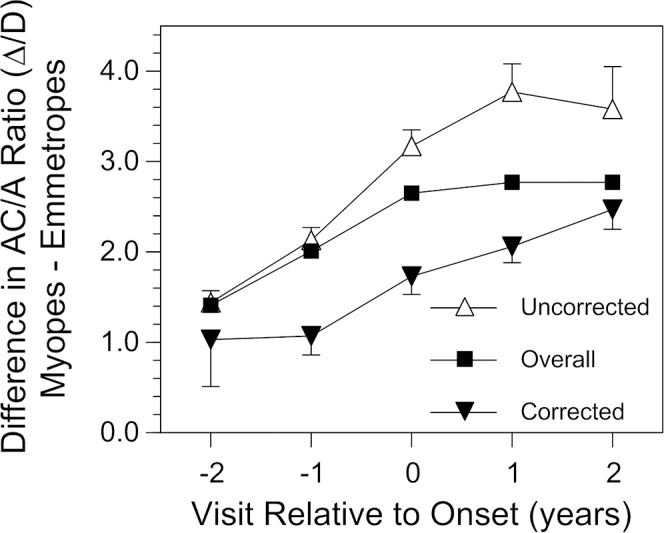
The difference in response AC/A ratio between children who became myopic and age-, sex-, and ethnicity-matched emmetropic values as a function of visit relative to the onset of myopia by refractive error correction status. Due to sample size considerations, only visits from −2 to +2 are plotted. All error bars are ±SEM.
Discussion
The response AC/A ratio was higher in children who became myopic compared to children who remained emmetropic 4 years prior to onset and in each year for 5 years after onset. The elevated AC/A ratios seen in myopic children in the current study are consistent with many previous reports,3,20–23 but extend the information about the behavior of the AC/A ratio by several years before and after the onset of myopia. Differences in the AC/A ratio 4 years prior to myopia onset appear as early as some other major risk factors. Specifically, previous analyses of CLEERE results found less hyperopic refractive errors and longer axial lengths in children who become myopic compared to those who remained emmetropic beginning 4 years and 3 years prior to onset, respectively.36 Although differences in refractive error and AC/A ratio between children who become myopic and those who remain emmetropic are both early signs of impending myopia, previous analysis of CLEERE data shows that the AC/A ratio does not add independent new information to the prediction of future myopia compared to a low hyperopic refractive error by itself.24 Putting these two ideas together, an increasing AC/A ratio appears to play a role in the process of becoming myopic, but in terms of prediction of onset, nothing predicts future myopia better than a child's current low hyperopic to emmetropic refractive error.
One of the more interesting aspects of this temporal pattern is the two phases relative to onset. Preonset, the AC/A ratio increased monotonically. The AC/A ratio reached its peak in the year of onset but did not continue to increase afterwards; the ratio was stable in all years postonset. This pattern of increase followed by stability is reminiscent of that for relative peripheral refraction in CLEERE data.36 One distinction is that relative peripheral refraction only became different relative to emmetropes −2 years before onset rather than −4 years for the AC/A ratio. Despite the mismatch in timing, both relative peripheral refraction and the AC/A ratio reached their peak values in the year of myopia onset and became stable, on average, after myopia onset. The question of whether these two features, accommodative convergence and ocular shape, share a common process requires further consideration.
One possible connection between the two may reside in the ciliary muscle. Myopes have been reported to have larger ciliary muscles, assessed as either greater in thickness or in cross-sectional area.37–40 Cultured smooth muscle isolated from a variety of tissues, including bladder, airway, and blood vessels, show hypertrophy, increased stiffness, and poorer contractility in response to mechanical stress.41–43 Ocular equatorial expansion may represent an analogous stress on ciliary smooth muscle in the eye. Perhaps a larger and stiffer ciliary muscle mass creates a distorting force on the growing eye while also compromising accommodative function. A higher AC/A ratio was associated with increased accommodative lag across most study visits (Table 2). One interpretation of this association is as an adaptive response to binocular stress from esophoria. However, this association may indicate a general compromise to accommodative function in the way that cycloplegia or presbyopia increases both the AC/A ratio and monocular accommodative lag.12–14,19 This “squeeze” phase preonset may be followed by a “freeze” phase postonset at a more stable plateau. This leveling off does not necessarily promote a stable refractive error if axial elongation continues without corresponding changes in crystalline lens or corneal power. This distorting, restrictive force has also been proposed as the reason for the “freeze” in the compensatory crystalline lens thinning, flattening, and power loss seen at the onset of myopia.44 Therefore, several accommodative, peripheral refractive, and crystalline lens optical findings in myopia might have a hypothesized common source: an increasing ciliary muscle dysfunction prior to onset that reaches a maximum at onset.
Consistent with an increasing AC/A ratio during a period of stable lag preonset,26 the proportion of children with near esophoria increased among those who became myopic compared to children who remained emmetropic. This proportion then decreased after onset. One reason for the decrease may be greater accommodative lag after myopia onset when the AC/A ratio was stable.26 Vergence adaptation is another possible source of a reduced amount of esophoria, although evidence suggests that myopes' vergence adaptation is either deficient or no different from that of other refractive error groups.45,46 Greater lag would be expected to reduce esophoria at near but the univariate logistic regression results suggest that near esophoria may be driven more by the AC/A ratio than the level of accommodative lag. In addition, measurements of lag in the current study were taken under monocular conditions. Greater accommodative lag has been found in individuals with esophoria compared to those with exophoria, but only under binocular conditions.47
Another source of the lower proportion of children with near esophoria after myopia onset might be adaptation to wearing glasses. More children wore glasses to testing as myopia progressed across study years (Table 1). The AC/A ratio was measured while children wore their habitual correction, which would mean with no correction if none was brought to testing. In contrast, cover testing was either done with the habitual spectacle or contact lens correction in place, or with retinoscopy findings in a trial frame if there was a clinically significant uncorrected ametropia. The AC/A ratio was likely unaffected by testing with or without a correction. Uncorrected myopia decreases the accommodative demand and has been shown to reduce accommodative lag using this study's protocol,26 but all children in the analysis accommodated at least 1 D and were within the range of linearity between accommodation and accommodative convergence.30 The AC/A ratio would be expected to be independent of lag within this range. Longer-term adaptation to wearing glasses, however, may affect cover test and AC/A ratio results. The AC/A ratio has been reported to be higher by approximately 0.75 Δ/D with a new full correction in previously undercorrected subjects compared to results 1 week later.48 The decrease over the week was attributed to the dissipation of habitually greater positive relative convergence while undercorrected rather than to changes in lag; there was no substantial difference in accommodative response between visits.48 Corrected subjects in the current study wore their spectacles or contact lenses to testing and were therefore likely to be adapted wearers. Subjects without spectacles or contact lenses had approximately −1.4 D of uncorrected myopia on average. The 1.0 to 1.5 Δ/D higher AC/A ratios in uncorrected children may well represent habitual use of greater positive relative convergence while uncorrected. Likewise, the decreasing proportion of children with near esophoria after myopia onset might represent less of this excess positive relative convergence in the larger number of children wearing and adapting to myopic corrections.
Young myopes have several characteristics suspected of promoting faster rates of myopia progression: increased amount of near work, greater accommodative lag, having near esophoria, and having a higher AC/A ratio. However, results in the literature have not provided consistent evidence in favor of a substantial role for these factors. For example, near work was related to the rate of myopia progression in one study of Norwegian engineering students,49 but showed inconsistently significant results with minimal clinical relevance to progression in CLEERE,50 and showed no association with progression in several studies from Asia.51–53 Results for accommodative lag are similarly inconsistent. One study found that an elevated accommodative lag was associated with myopia progression in adults,54 but another found that children with higher than the median lag had only 0.24 D more progression in 3 years,55 while others have found no association in children56,57 or that lower accommodative lag is associated with myopia progression in adults.58 More rapid myopia progression is often associated with esophoria,59,60 but progression has also been no different when compared to exophoria,55 or even minimally greater for children with exophoria.61 An elevated AC/A ratio was associated with faster progression in one study of 95 children over 2 years.57 However, the current study showed no association between the AC/A ratio and the rate of myopia progression regardless of the level of near work activity.
Strengths of this study are the large size and the ethnic diversity of the sample. The length of follow-up was extensive, allowing comparisons to be made over a long period of time from −5 years prior to myopia onset to +5 years after. Annual observation made the onset of myopia detectable within 1 year of the event. A study limitation was that AC/A ratio data were not collected in study years prior to 1995, reducing the number of children with both refractive error and AC/A ratio data across visits. The AC/A ratio measurement was made without knowledge of or adjustment for the day's prior near work demand, thereby excluding any variation in AC/A ratio that might have occurred with adaptation to a sustained near task. Variation in near work over the course of the year or prior to testing was also unknown because the visual activity survey was only given once per year. Another limitation is that the data do not include measurement of myopic children both with and without their myopic correction. Correction status during testing depended on the availability of a correction and the child's decision to wear it or not on the day of testing. Lastly, measurements of accommodative response as part of the AC/A ratio testing required opening of the vergence loop and therefore were monocular.
In summary, the response AC/A ratio increased in children who became myopic compared to children who remained emmetropic as early as −4 years prior to myopia onset. The AC/A ratio reached its peak at myopia onset and remained elevated and stable through at least +5 years after onset. A higher AC/A ratio was likely responsible for the increased proportion of near esophoria in children who became myopic. An elevated AC/A ratio was associated with increased accommodative lag but was not associated with an increased rate of myopic progression regardless of the amount of near work. The association with increased accommodative lag suggests that the more likely source of the higher AC/A ratio in myopic children is compromised accommodation requiring an increased effort needed per diopter of accommodative output rather than an increase in the neural accommodative convergence crosslink gain relationship.
Acknowledgments
Supported by National Institutes of Health/National Eye Institute (NEI) grants U10-EY08893, R24-EY014792, and R21-EY012273, the Ohio Lions Eye Research Foundation, and the E.F. Wildermuth Foundation.
Disclosure: D.O. Mutti, None; G.L. Mitchell, None; L.A. Jones-Jordan, None; S.A. Cotter, None; R.N. Kleinstein, None; R.E. Manny, None; J.D. Twelker, None; K. Zadnik, None
Appendix
The members of the CLEERE Study Group include:
Clinical Centers
Franklin Primary Health Center, Inc.: Sandral Hullett, MD MPH (Principal Investigator, 1997–2007), Robert N. Kleinstein, OD MPH PhD (Co-Investigator, 1997–2007), Janene Sims, OD (Optometrist, 1997–2001 and 2004–2007), Raphael Weeks, OD (Optometrist, 1999–2007), Sandra Williams (Study Coordinator, 1999–2007), LeeAndra Calvin (Study Coordinator, 1997–1999), Melvin D. Shipp, OD MPH DrPH (Co-Investigator, 1997–2004). Drs. Kleinstein and Sims are affiliated with the University of Alabama at Birmingham School of Optometry.
University of California, Berkeley School of Optometry, Berkeley, CA: Nina E. Friedman, OD MS (Principal Investigator, 1999–2001), Pamela Qualley, MA (Study Coordinator, 1997–2001), Donald O. Mutti, OD PhD (Principal Investigator, 1996–1999), Karla Zadnik, OD PhD (Optometrist, 1996–2001).
University of Houston College of Optometry: Ruth E. Manny, OD PhD (Principal Investigator, 1997–2007), Suzanne M. Wickum, OD (Optometrist, 1999–2007), Ailene Kim, OD (Optometrist, 2003–2007), Bronwen Mathis, OD (Optometrist, 2002–2007), Mamie Batres (Study Coordinator, 2004–2007). Sally Henry (Study Coordinator, 1997–1998), Janice M. Wensveen, OD PhD (Optometrist, 1997–2001), Connie J. Crossnoe, OD (Optometrist, 1997–2003), Stephanie L. Tom, OD (Optometrist, 1999–2002), Jennifer A. McLeod (Study Coordinator, 1998–2004), Julio C. Quiralte (Study Coordinator, 1998–2005), Gaby Solis (Study Coordinator, 2005–2007).
Southern California College of Optometry, Fullerton, CA: Susan A. Cotter, OD (Principal Investigator, 2004–2007, Optometrist, 1997–2004), Julie A. Yu, OD (Principal Investigator, 1997–2004; Optometrist 2005–2007), Raymond J. Chu, OD (Optometrist, 2001–2007), Carmen N. Barnhardt, OD, MS (Optometrist 2004–2007), Jessica Chang, OD (Optometrist, 2005–2007), Kristine Huang, OD (Optometrist, 2005–2007), Rebecca Bridgeford (Study Coordinator, 2005–2006), Connie Chu, OD (Optometrist, 2004–2005), Soonsi Kwon, OD (Optometrist, 1998–2004), Gen Lee (Study Coordinator, 1999–2003), John Lee, OD (Optometrist, 2000–2003), Robert J. Lee, OD (Optometrist, 1997–2001), Raymond Maeda, OD (Optometrist, 1999–2003), Rachael Emerson (Study Coordinator, 1997–1999); Tracy Leonhardt (Study Coordinator, 2003–2004).
University of Arizona, Department of Ophthalmology, Tucson, AZ: J. Daniel Twelker, OD PhD (Principal Investigator, 2000–2010), Dawn Messer, OD (Optometrist, 2000–2010), Denise Flores (Study Coordinator, 2000–2007, Rita Bhakta, OD (Optometrist, 2000–2004), Katie Garvey, OD (Optometrist, 2006–2010).
Resource Centers
Chairman's Office, The Ohio State University College of Optometry, Columbus, OH: Karla Zadnik, OD PhD (Chairman, 1997-present), Jodi M. Malone, RN (Study Coordinator, 1997–2010).
Videophakometry Reading Center, The Ohio State University College of Optometry, Columbus, OH: Donald O. Mutti, OD PhD (Director, 1997–present), Vidya Subramanian, MS (Reader, 2006–), Huan Sheng, MD MS (Reader, 2000–2006), Holly Omlor (Reader, 2003–2006), Meliha Rahmani (Reader, 2004–2006), Jaclyn Brickman (Reader, 2002–2003), Amy Wang (Reader, 2002–2003), Philip Arner (Reader, 2002–2004), Samuel Taylor (Reader, 2002–2003), Myhanh T. Nguyen (Reader, 1998–2001), Terry W. Walker (Reader, 1997–2001).
Optometry Coordinating Center, The Ohio State University College of Optometry, Columbus, OH: Lisa A. Jones-Jordan, PhD (Director, 1997–present), Linda Barrett (Data Entry Operator, 1997–2008), John Hayes, PhD (Biostatistician, 2001–2007), G. Lynn Mitchell, MAS Biostatistician, 1998–present), Melvin L. Moeschberger, PhD (Consultant, 1997–2010), Loraine Sinnott, PhD (Biostatistician, 2005–present), Pamela Wessel (Program Coordinator, 2000–present), Julie N. Swartzendruber, MA (Program Coordinator, 1998–2000).
Project Office, National Eye Institute, Rockville, MD: Donald F. Everett, MA.
Committees
Executive Committee: Karla Zadnik, OD PhD (Chairman), Lisa A. Jones-Jordan, PhD, Robert N. Kleinstein, OD MPH PhD, Ruth E. Manny, OD PhD, Donald O. Mutti, OD PhD, J. Daniel Twelker, OD PhD, Susan A. Cotter, OD.
References
- 1. Schor CM,, Glenn A. The Fry award lecture: adaptive regulation of accommodative vergence and vergence accommodation. Am J Optom Physiol Opt. 1986; 63: 587–609. [PubMed] [Google Scholar]
- 2. Turner JE,, Horwood AM,, Houston SM,, Riddell PM. Development of the response AC/A ratio over the first year of life. Vision Res. 2002; 42: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 3. Mutti DO,, Jones LA,, Moeschberger ML,, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000; 41: 2469–2478. [PubMed] [Google Scholar]
- 4. Bharadwaj SR,, Candy TR. Cues for the control of ocular accommodation and vergence during postnatal human development. J Vis. 2008; 8 16: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiagarajan P,, Lakshminarayanan V,, Bobier WR. Effect of vergence adaptation and positive fusional vergence training on oculomotor parameters. Optom Vis Sci. 2010; 87: 487–493. [DOI] [PubMed] [Google Scholar]
- 6. Rainey BB. The effect of prism adaptation on the response AC/A ratio. Ophthal Physiol Opt. 2000; 20: 199–206. [PubMed] [Google Scholar]
- 7. Brautaset RL,, Jennings AJ. Effects of orthoptic treatment on the CA/C and AC/A ratios in convergence insufficiency. Invest Ophthalmol Vis Sci. 2006; 47: 2876–2880. [DOI] [PubMed] [Google Scholar]
- 8. Flom MC. On the relationship between accommodation and accommodative convergence. III. Effects of orthoptics. Am J Optom Arch Am Acad Optom. 1960; 37: 619–632. [DOI] [PubMed] [Google Scholar]
- 9. Jiang BC,, Ramamirtham R. The adaptive effect of narrowing the interocular separation on the AC/A ratio. Vision Res. 2005; 45: 2704–2709. [DOI] [PubMed] [Google Scholar]
- 10. Judge SJ. Optically-induced changes in tonic vergence and AC/A ratio in normal monkeys and monkeys with lesions of the flocculus and ventral paraflocculus. Exp Brain Res. 1987; 66: 1–9. [DOI] [PubMed] [Google Scholar]
- 11. Miles FA,, Judge SJ,, Optican LM. Optically induced changes in the couplings between vergence and accommodation. J Neurosci. 1987; 7: 2576–2589. [PMC free article] [PubMed] [Google Scholar]
- 12. Breinin GM,, Chin NB. Accommodation, convergence and aging. Doc Ophthalmol. 1973; 34: 109–121. [DOI] [PubMed] [Google Scholar]
- 13. Bruce AS,, Atchison DA,, Bhoola H. Accommodation-convergence relationships and age. Invest Ophthalmol Vis Sci. 1995; 36: 406–413. [PubMed] [Google Scholar]
- 14. Ciuffreda KJ,, Rosenfield M,, Chen HW. The AC/A ratio, age and presbyopia. Ophthal Physiol Opt. 1997; 17: 307–315. [PubMed] [Google Scholar]
- 15. Anderson HA,, Hentz G,, Glasser A,, Stuebing KK,, Manny RE. Minus-lens-stimulated accommodative amplitude decreases sigmoidally with age: a study of objectively measured accommodative amplitudes from age 3. Invest Ophthalmol Vis Sci. 2008; 49: 2919–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richdale K,, Sinnott LT,, Bullimore MA,, et al. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Invest Ophthalmol Vis Sci. 2013; 54: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamm E,, Croft MA,, Jungkunz W,, Lutjen-Drecoll E,, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey. Role of the choroid. Arch Ophthalmol. 1992; 110: 871–876. [DOI] [PubMed] [Google Scholar]
- 18. Fisher RF. The ciliary body in accommodation. Trans Ophthalmol Soc UK. 1986; 105 pt 2: 208–219. [PubMed] [Google Scholar]
- 19. Christoferson KW,, Ogle KN. The effect of homatropine on the accommodation-convergence association. Arch Ophthalmol. 1956; 55: 779–791. [DOI] [PubMed] [Google Scholar]
- 20. Gwiazda J,, Grice K,, Thorn F. Response AC/A ratios are elevated in myopic children. Ophthal Physiol Opt. 1999; 19: 173–179. [DOI] [PubMed] [Google Scholar]
- 21. Jiang BC. Parameters of accommodative and vergence systems and the development of late-onset myopia. Invest Ophthalmol Vis Sci. 1995; 36: 1737–1742. [PubMed] [Google Scholar]
- 22. Rosenfield M,, Gilmartin B. Effect of a near-vision task on the response AC/A of a myopic population. Ophthal Physiol Opt. 1987; 7: 225–233. [PubMed] [Google Scholar]
- 23. Gwiazda J,, Thorn F,, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005; 82: 273–278. [DOI] [PubMed] [Google Scholar]
- 24. Zadnik K,, Sinnott LT,, Cotter SA,, et al. Prediction of juvenile-onset myopia. JAMA Ophthalmol. 2015; 133: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gwiazda J,, Thorn F,, Bauer J,, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993; 34: 690–694. [PubMed] [Google Scholar]
- 26. Mutti DO,, Mitchell GL,, Hayes JR,, et al. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006; 47: 837–846. [DOI] [PubMed] [Google Scholar]
- 27. Twelker JD,, Mitchell GL,, Messer DH,, et al. Children's ocular components and age, gender, and ethnicity. Optom Vis Sci. 2009; 86: 918–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zadnik K,, Mutti DO,, Friedman NE,, et al. Ocular predictors of the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 1999; 40: 1936–1943. [PubMed] [Google Scholar]
- 29. Jones LA,, Mitchell GL,, Zadnik K;, The CLEERE Study Group. Agreement between parent-reported and clinician-assessed race in the CLEERE Study. Control Clin Trials. 2001; 22: 98S. [Google Scholar]
- 30. Flom M. On the relationship between accommodation and accommodative convergence. Part I. Linearity. Am J Optom Arch Am Acad Optom. 1960; 37: 474–482. [PubMed] [Google Scholar]
- 31. Joubert C,, Bedell HE. Proximal vergence and perceived distance. Optom Vis Sci. 1990; 67: 29–35. [DOI] [PubMed] [Google Scholar]
- 32. Seddon JM,, Sahagian CR,, Glynn RJ,, et al. Evaluation of an iris color classification system. Invest Ophthalmol Vis Sci. 1990; 31: 1592–1598. [PubMed] [Google Scholar]
- 33. Kleinstein RN,, Mutti DO,, Manny RE,, Shin JA,, Zadnik K. Cycloplegia in African-American children. Optom Vis Sci. 1999; 76: 102–107. [DOI] [PubMed] [Google Scholar]
- 34. Bozdogan H. Model selection and Akaike's Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika. 1987; 52: 345–370. [Google Scholar]
- 35. Zadnik K,, Mutti DO,, Mitchell GL,, et al. Normal eye growth in emmetropic schoolchildren. Optom Vis Sci. 2004; 81: 819–828. [DOI] [PubMed] [Google Scholar]
- 36. Mutti DO,, Hayes JR,, Mitchell GL,, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007; 48: 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bailey MD,, Sinnott LT,, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008; 49: 4353–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oliveira C,, Tello C,, Liebmann JM,, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005; 140: 324–325. [DOI] [PubMed] [Google Scholar]
- 39. Sheppard AL,, Davies LN. In vivo analysis of ciliary muscle morphological changes with accommodation and axial ametropia. Invest Ophthalmol Vis Sci. 2010; 51: 6882–6889. [DOI] [PubMed] [Google Scholar]
- 40. Buckhurst H,, Gilmartin B,, Cubbidge RP,, Nagra M,, Logan NS. Ocular biometric correlates of ciliary muscle thickness in human myopia. Ophthal Physiol Opt. 2013; 33: 294–304. [DOI] [PubMed] [Google Scholar]
- 41. Deng L,, Fairbank NJ,, Fabry B,, Smith PG,, Maksym GN. Localized mechanical stress induces time-dependent actin cytoskeletal remodeling and stiffening in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2004; 287: C440–C448. [DOI] [PubMed] [Google Scholar]
- 42. Ren J,, Albinsson S,, Hellstrand P. Distinct effects of voltage- and store-dependent calcium influx on stretch-induced differentiation and growth in vascular smooth muscle. J Biol Chem. 2010; 285: 31829–31839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roby T,, Olsen S,, Nagatomi J. Effect of sustained tension on bladder smooth muscle cells in three-dimensional culture. Ann Biomed Eng. 2008; 36: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 44. Mutti DO,, Mitchell GL,, Sinnott LT,, et al. Corneal and crystalline lens dimensions before and after myopia onset. Optom Vis Sci. 2012; 89: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. North RV,, Sethi B,, Owen K. Adaptation ability of subjects with different refractive errors. Optom Vis Sci. 1989; 66: 296–299. [DOI] [PubMed] [Google Scholar]
- 46. Rosenfield M,, Gilmartin B. Accommodative adaptation induced by sustained disparity-vergence. Am J Optom Physiol Opt. 1988; 65: 118–126. [DOI] [PubMed] [Google Scholar]
- 47. Sreenivasan V,, Irving EL,, Bobier WR. Effect of heterophoria type and myopia on accommodative and vergence responses during sustained near activity in children. Vision Res. 2012; 57: 9–17. [DOI] [PubMed] [Google Scholar]
- 48. Flom MC,, Takahashi E. The AC/A ratio and undercorrected myopia. Am J Optom Arch Am Acad Optom. 1962; 39: 305–312. [PubMed] [Google Scholar]
- 49. Kinge B,, Midelfart A,, Jacobsen G,, Rystad J. The influence of near-work on development of myopia among university students. A three-year longitudinal study among engineering students in Norway. Acta Ophthalmol Scand. 2000; 78: 26–29. [DOI] [PubMed] [Google Scholar]
- 50. Jones-Jordan LA,, Sinnott LT,, Cotter SA,, et al. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012; 53: 7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saw SM,, Nieto FJ,, Katz J,, et al. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000; 77: 549–554. [DOI] [PubMed] [Google Scholar]
- 52. Tan NW,, Saw SM,, Lam DS,, et al. Temporal variations in myopia progression in Singaporean children within an academic year. Optom Vis Sci. 2000; 77: 465–472. [DOI] [PubMed] [Google Scholar]
- 53. Saw SM,, Chua WH,, Gazzard G,, et al. Eye growth changes in myopic children in Singapore. Br J Ophthalmol. 2005; 89: 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allen PM,, O'Leary DJ. Accommodation functions: co-dependency and relationship to refractive error. Vision Res. 2006; 46: 491–505. [DOI] [PubMed] [Google Scholar]
- 55. Gwiazda J,, Hyman L,, Hussein M,, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003; 44: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 56. Weizhong L,, Zhikuan Y,, Wen L,, Xiang C,, Jian G. A longitudinal study on the relationship between myopia development and near accommodation lag in myopic children. Ophthal Physiol Opt. 2008; 28: 57–61. [DOI] [PubMed] [Google Scholar]
- 57. Price H,, Allen PM,, Radhakrishnan H,, et al. The Cambridge anti-myopia study: variables associated with myopia progression. Optom Vis Sci. 2013; 90: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 58. Rosenfield M,, Desai R,, Portello JK. Do progressing myopes show reduced accommodative responses? Optom Vis Sci. 2002; 79: 268–273. [DOI] [PubMed] [Google Scholar]
- 59. Fulk GW,, Cyert LA,, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000; 77: 395–401. [DOI] [PubMed] [Google Scholar]
- 60. Goss DA,, Jackson TW. Clinical findings before the onset of myopia in youth: 4. Parental history of myopia. Optom Vis Sci. 1996; 73: 279–282. [DOI] [PubMed] [Google Scholar]
- 61. Berntsen DA,, Sinnott LT,, Mutti DO,, Zadnik K;, The CLEERE Study Group. Accommodative lag and juvenile-onset myopia progression in children wearing refractive correction. Vision Res. 2011; 51: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]