Introduction
Vitiligo is a common, disfiguring autoimmune disease that negatively affects patients’ self-esteem and quality of life (1, 2). Existing vitiligo treatments, which are used off-label, are general, non-targeted immunosuppressants that provide only modest efficacy. Developing safe and effective treatments requires a better understanding of disease pathogenesis to identify new therapeutic targets (3). Vitiligo is caused by a dynamic interplay between genetic and environmental risks that initiates an autoimmune attack on melanocytes in the skin.
Vitiligo pathogenesis
Genetics
The observation that vitiligo was more prevalent in the immediate relatives of patients with vitiligo provided early evidence of its heritability. While vitiligo affects ~1% of the general population (4), the risk of a patient’s sibling developing the disease is 6%, and for an identical twin it is 23% (5). In addition, patients with vitiligo and their relatives have an increased risk of developing other autoimmune diseases, including autoimmune thyroiditis, type 1 diabetes, pernicious anemia, and Addison’s disease, suggesting that vitiligo is also an autoimmune disease (6). These early observations were later confirmed by genome-wide association (GWA) studies, which identified numerous common genetic variants in vitiligo patients encoding for components of both the innate (NLRP1, IFIH1, CASP7, C1QTNF6, TRIF) and adaptive immune system (FOXP3, BACH2, CD80, CCR6, PTPN22, IL2R, alpha GZMB, HLA class I and II) [(7–9), see also Spritz R, Andersen G: Genetics of vitiligo; in this issue].
Oxidative Stress
Accumulating evidence suggests that melanocytes from vitiligo patients have intrinsic defects that reduce their capacity to manage cellular stress {reviewed in (10)}. Epidermal cells, including melanocytes, are constantly exposed to environmental stressors such as UV radiation and various chemicals, which can increase production of reactive oxygen species (ROS). While healthy melanocytes are capable of mitigating these stressors, melanocytes from vitiligo patients appear to be more vulnerable. For example, melanocytes from perilesional vitiligo skin demonstrate a dilated endoplasmic reticulum (ER) and abnormalities in their mitochondria and melanosome structure, all of which are characteristic of elevated cellular stress. High concentrations of epidermal H2O2 level and a decreased level of catalase, a critical enzyme that protects cells from oxidative damage, have been observed in skin of patients with vitiligo (11–17).
Environment
The earliest triggering events that lead to vitiligo are not fully understood. Multiple studies suggest that a combination of melanocyte intrinsic defects and exposure to specific environmental factors may play a central role in disease onset. This was evident in a group of factory workers who developed vitiligo following exposure to monobenzone, an organic chemical phenol, in their gloves (18). Later studies confirmed that a history of exposure to other phenolic and catecholic chemicals found in dyes (especially hair dyes), resins/adhesives, and leather was associated with vitiligo (19, 20).
Melanogenesis is a multi-step process through which the melanocyte produces melanin. Tyrosinase is a rate-limiting enzyme in this process that controls the production of melanin through oxidation of the amino acid tyrosine, a naturally occurring phenol {reviewed in (21) and further discussed in this issue, Harris JE: Chemical-induced vitiligo}. In vitro studies demonstrated that chemical phenols can act as tyrosine analogs within the melanocyte, precipitating high levels of cellular stress. This may include increased production of ROS and triggering of the unfolded protein response (UPR), which in turn activates innate inflammation (22, 23).
Innate Immunity
As mentioned earlier, GWA studies in vitiligo patients implicated multiple susceptibility loci related to the genes that control the innate immunity (7–9). This likely causes dysregulated innate activation in response to melanocyte stress, demonstrated through recruitment of innate populations like natural killer (NK) cells and production and release of high levels of pro-inflammatory proteins and cytokines including heat-shock proteins (HSP), IL-1β, IL6, and IL-8 {(22–28), and reviewed in (10, 29)}. Among larger HSP molecules, inducible HSP70 (HSP70i) is unique, as it can be secreted to chaperone peptides specific to the originating host cells (30). Recently, HSP70i has been shown to be important for vitiligo pathogenesis in a mouse model through induction of inflammatory dendritic cells (DCs), which themselves may be cytotoxic or carry and present melanocyte-specific antigens to T cells in lymphoid tissues (24, 25). This has been proposed to be a key crosstalk step between innate and adaptive immunity leading to the T cell-mediated autoimmune destruction of melanocytes (31).
Adaptive Immunity
Ultimately, cytotoxic CD8+ T cells are responsible for the destruction of melanocytes (32). Cytokines secreted within the skin act as an early signal to help these autoreactive T cells locate stressed melanocytes. This is probably important because the epidermis is not vascularized, and so active mechanisms are required to help them efficiently locate melanocytes (33). Chemokines are small, secreted proteins that act as chemoattractants to guide T cell migration. IFN-γ and IFN-γ-induced chemokines (CXCL9 and CXCL10) are highly expressed in the skin and blood of patients with vitiligo, as well as a mouse model (34–36). In addition, IFN-γ and CXCL10 are required for both disease progression and maintenance in a mouse model of the disease (34, 37). Recently, a separate study demonstrated that serum CXCL10 was not only higher in patients with vitiligo compared to healthy controls, but its level was associated with disease activity and significantly decreased after successful treatment, suggesting it may be used as a biomarker to monitor the disease activity and treatment response (36).
Emerging treatments
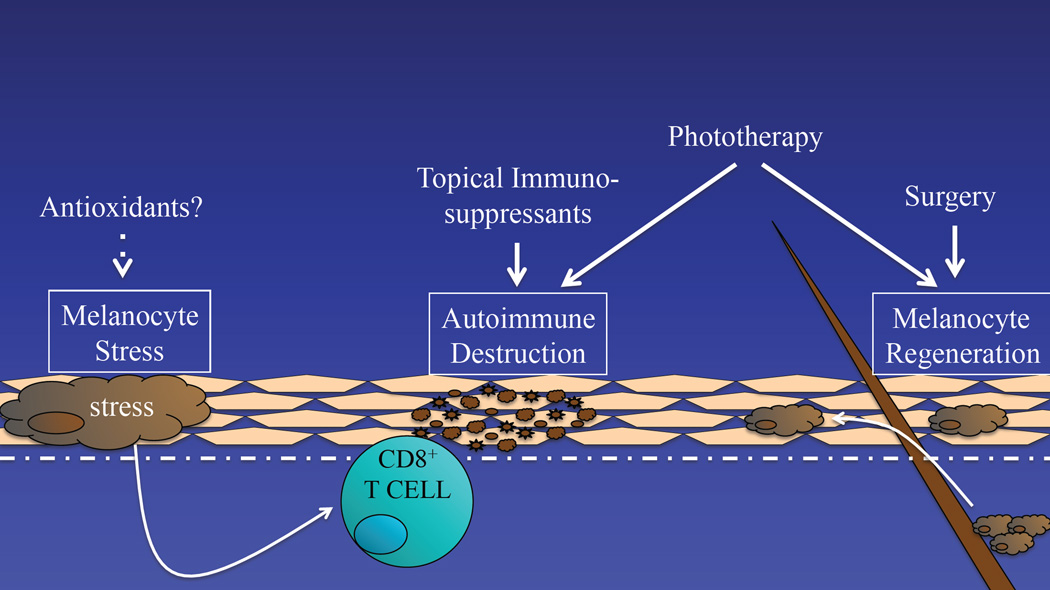
Based on our current understanding of vitiligo pathogenesis, a successful strategy to treat vitiligo should incorporate three distinct approaches: reducing melanocytes stress, regulating the autoimmune response, and stimulating melanocyte regeneration. Existing treatments partially address these needs, however emerging therapies may do this in a more targeted way, and combination therapies may synergize to produce a better overall response (Fig. 1).
Fig. 1.
Vitiligo pathogenesis begins with altered melanocytes that exhibit an elevated cellular stress response. This triggers autoimmunity, which targets melanocytes for destruction, resulting in focal depigmentation. Repigmentation requires the growth and migration of melanocytes, typically from hair follicles. Thus, there are 3 goals to consider during the treatment of vitiligo: 1) reducing melanocyte stress, 2) suppressing autoimmune targeting of melanocytes, and 3) promoting melanocyte regeneration. Current treatments, including topical immunosuppressants, phototherapy, and surgical approaches, partially address these goals in overall non-targeted ways.
Reducing melanocyte stress
The apparent reduction of catalase enzyme in the epidermis of vitiligo patients as well as elevated levels of ROS in lesional skin prompted the hypothesis that treating patients with antioxidants or otherwise controlling ROS might be an effective treatment strategy (38). Pseudocatalase describes a treatment cream comprised of any number of metal ions capable of converting H2O2, a common ROS, into water and oxygen. Early studies using pseudocatalase combined with phototherapy for vitiligo patients seemed promising (38–40), however they were either not controlled or not blinded, and subsequent studies have not reproduced positive results (41–43). It is unclear whether this strategy could be optimized or otherwise improved for the development of future therapies.
Oral or topical natural health products, vitamins, and supplements have been suggested as possible therapies based on their antioxidant and anti-inflammatory properties {(44), and reviewed in this issue, Grimes PE, Nashawati R: The role of diet and supplements in vitiligo management}. The herbal supplement Gingko biloba has been tested in 2 small trials and reported to promote some improvement (45, 46). The plant extract Polypodium leucotomos reportedly improved responses to nbUVB in a small group of vitiligo patients compared to placebo (47). One group tested nbUVB with or without supplementation by an “antioxidant pool” that included α-lipoic acid, vitamin C, vitamin E, and polyunsaturated fatty acids. They reported greater efficacy in patients who received a combination of nbUVB plus antioxidants (48). Larger, controlled trials will need to be conducted to determine if adding antioxidants will be a beneficial strategy to add to patient management.
Regulating autoimmunity
Over the past decade, significant progress has been made in the development of immunomodulators to treat inflammatory skin disease, including more targeted treatments. Recent advances in our understanding of the immunopathogenesis of vitiligo have helped us to identify novel immune targets to develop and test new vitiligo treatments.
HSP70i
One group reported a role for the heat shock protein HSP70i in vitiligo pathogenesis, suggesting that it was released by stressed melanocytes and initiated innate inflammation within the skin (49). They then found that mutating the protein made it less immunogenic, and appeared to even induce tolerance when expressed in the skin of a mouse model, preventing the onset of disease. They proposed future testing this as a new treatment for vitiligo (25), although DNA delivery of a mutant protein in patient skin may take some time to develop and demonstrate safety.
IFN-γ/CXCL10
We previously reported that the IFN-γ/CXCL10 axis is a critical signaling pathway required for both the progression and maintenance of vitiligo, and hypothesized that targeting this pathway could be an effective treatment strategy (34, 37). A variety of antibodies and small-molecule inhibitors have already been developed to target components of this pathway (including IFN-γ, CXCL10, and the CXCL10 receptor CXCR3), and were found to be safe in early phase clinical trials for treatment of other autoimmune diseases including psoriasis, rheumatoid arthritis, and Crohn’s disease. Most of these trials failed to reach their efficacy endpoint, likely because IFN-γ is not a major driving cytokine in those diseases. However, recent findings in patients and a mouse model suggest that vitiligo is an optimal disease to test those investigational drugs (3).
JAK-STAT signaling
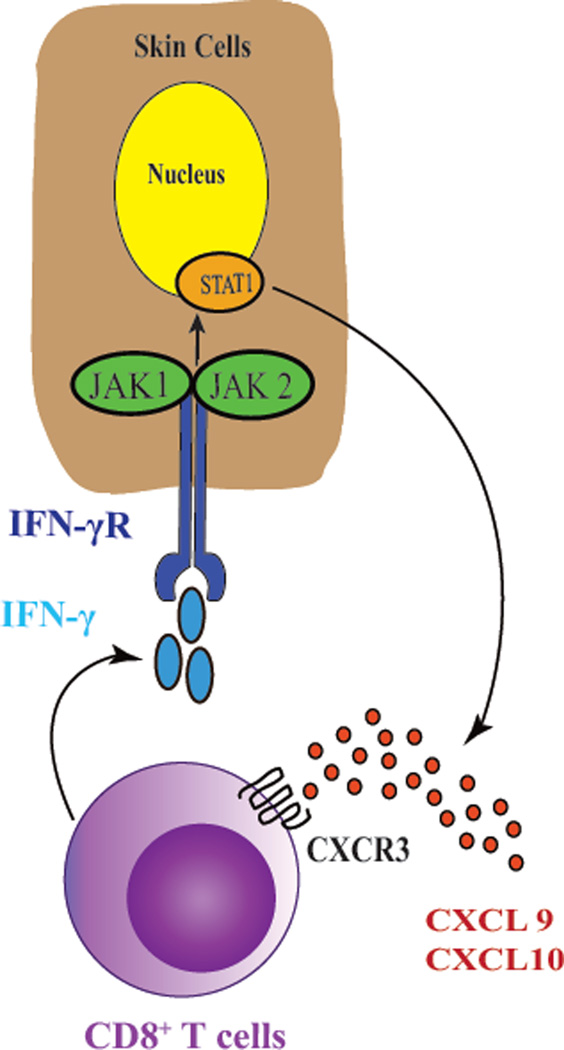
JAK-STAT signaling is essential to transmit extracellular signals of many cytokines, including IFN-γ, to the nucleus. Following ligation of the cytokine receptor, Janus kinases (JAKs) phosphorylate signal transducer and activator of transcription proteins (STATs), which become activated and induce transcription of target genes. There are four members of JAK family, including JAK1, JAK2, JAK3, and Tyrosine kinase 2 (TYK2). Among these, JAK1 and JAK2 are directly involved in IFN-γ signaling, which activate STAT1 and thus induce the transcription of IFN-γ-induced genes, including CXCL10 {(Fig. 2), reviewed in (50)}.
Fig. 2.
Autoimmunity in vitiligo is driven by the IFN-γ-CXCL10 cytokine signaling pathway. Activated melanocyte-specific CD8+ T cells secrete IFN-γ, which signals through the IFN-γ receptor (IFN-γR) to activate JAK1/2 and STAT1. This induces the production of CXCL9 and CXCL10, which signal through their receptor CXCR3 to recruit more autoreactive T cells to the epidermis, resulting in widespread melanocyte destruction. Targeting this cytokine pathway represents an emerging treatment strategy for vitiligo.
Several small-molecule JAK inhibitors with distinct selectivity have been tested in patients or are under development. Interestingly, a patient with generalized vitiligo was reported to respond to treatment with oral tofacitinib, a JAK 1/3 inhibitor approved for the treatment of moderate to severe rheumatoid arthritis (51). Ruxolitinib, another JAK inhibitor with JAK 1/2 selectivity, is currently approved by the FDA for the treatment of intermediate or high-risk myelofibrosis and polycythemia vera (52, 53). We reported that a patient with vitiligo developed rapid repigmentation on his face and trunk after initiating oral ruxolitinib (54). The treatment response from these inhibitors did not appear to be durable, as patients lost the repigmentation after discontinuing treatment [(55) and Dr. B. King, personal communication].
Similar to all other immunosuppressive drugs, tofacitinib and ruxolitinib may have adverse effects, including opportunistic infections and rare malignancies. In addition, ruxolitinib may induce blood abnormalities including thrombocytopenia, anemia, and neutropenia (56). Topical formulation of these drugs may provide therapeutic benefit without increasing the risk of adverse events (57). Currently, an open label, phase 2, proof of concept clinical trial is recruiting participants to test the efficacy of topical ruxolitinib 1.5% in the treatment of vitiligo (58).
In addition to JAK inhibitors, STAT inhibitors could potentially have similar effects. So far, seven members have been identified in this family, however only STAT1 as a homodimer has been implicated in IFN-γ signaling {reviewed in (50)}. A previous study in vitro reported that statins, which lower cholesterol via inhibition of HMG-CoA reductase, inhibited STAT1 function (59). In addition, a vitiligo patient was reported to improve after taking oral simvastatin (60). A recent study tested systemic simvastatin in a mouse model of vitiligo and found it to be effective in both preventing and reversing disease (61). However, a small pilot clinical trial we conducted to test the efficacy of high-dose (80mg daily) oral simvastatin in patients with generalized vitiligo did not reach its primary efficacy end-point (62). Adverse effects of simvastatin limit dosing in humans, which may be responsible for the disparate results between the mouse model and vitiligo patients. An ongoing study is currently recruiting patients to evaluate the benefits of combining atorvastatin and UVB for the treatment of active vitiligo (63), and future studies could test topical simvastatin as a way to increase local concentrations without toxicity.
Immune checkpoints
Successful application of immunotherapy to treat metastatic melanoma via blockade of inhibitory checkpoints has gained recent attention. Immune checkpoints are molecules that modulate T cell responses to inflammation, and include Cytotoxic T-Lymphocyte-Associated protein 4 (CTLA-4) and Programmed cell death protein 1 (PD-1), among others (64). Interestingly, the treatment response of melanoma patients to immune checkpoint inhibitors correlates with the development of vitiligo (65). Some have hypothesized that activating these surface receptors could restore tolerance in vitiligo patients (66).
Abatacept is a fusion protein composed of the Fc region of the immunoglobulin IgG1 fused to the extracellular domain of CTLA-4. It is currently approved by the FDA for the treatment of moderate to severe rheumatoid arthritis (67). Recently, an open label, single arm, pilot study was initiated to test the efficacy of abatacept in patients with vitiligo (68). Additionally, PD-1 ligand (PD-L1, a PD-1 agonist) is currently under development, and is being tested in preclinical phases of inflammatory bowel disease and psoriasis (69).
Stimulating melanocyte regeneration
α-MSH
Phototherapy is currently first-line therapy for vitiligo, especially in patients with widespread disease {(1, 2) and reviewed in this issue, Esmat S, Hegazy RA, Shalaby S, Hu SC, et al: Phototherapy and combination therapies for vitiligo}. While the mechanism of its therapeutic effects are not completely understood, repigmentation from phototherapy is probably due to its ability to induce immunosuppression, but also to the induction of melanocyte stem cell differentiation and proliferation (70). Alpha-Melanocyte-stimulating hormone (α-MSH) is a naturally occurring hormone that stimulates melanogenesis {reviewed in (71)}. Afamelanotide, a synthetic analogue of αMSH, is currently approved by the European Medicines Agency to mitigate photosensitivity in erythropoietic protoporphyria (72), and thus may also improve the efficacy of phototherapy for vitiligo (73). Recently, a randomized comparative multicenter trial was conducted to test the safety and efficacy of an afamelanotide subcutaneous implant in combination with NB-UVB in adults with generalized vitiligo. The combination therapy was somewhat well-tolerated, although side effects included nausea and skin hyperpigmentation, which led some subjects to withdraw from the trial. The treatment resulted in faster and increased total repigmentation compared to NB-UVB monotherapy. This response was most evident in patients with darker skin (Fitzpatrick type IV to VI) (74). It is currently unknown whether afamelanotide monotherapy would have any benefit in the treatment of vitiligo.
WNT signaling
A recent study reported that melanocytes from vitiligo patients had defective WNT signaling, a pathway that promotes the differentiation of melanocyte precursors in skin. They hypothesized that this impaired signaling contributed to disease pathogenesis and, in particular, inhibited melanocyte regeneration and repigmentation during treatment. Studies using explanted human skin ex vivo suggested that WNT activators could enhance melanocyte differentiation (35). Thus, therapeutic WNT activation could potentially serve as an adjunctive therapy for vitiligo that supports melanocyte regeneration (75).
Selective sunscreen
Despite being the most effective current treatment for vitiligo, patient access to phototherapy is a challenge. To receive therapy, patients typically attend a specialized clinic two to three times weekly for up to 1–2 years in order to achieve a satisfactory response. While sun exposure is an inexpensive alternative to phototherapy, it is difficult to monitor exposure, and non-therapeutic wavelengths of light can be erythematogenic and dose-limiting. Recently, a topical formulation of dimethicone 1% was reported to selectively block wavelengths of sunlight below 300 nm, permitting therapeutic wavelengths in the nbUVB range (~311–312 nm) to penetrate. A small double-blind placebo-controlled study found that application of this cream followed by sun exposure was safe and effective at inducing repigmentation in lesional skin (76). However, this needs to be confirmed in larger clinical trials, and as the authors acknowledged its use would be limited by inadequate sunlight during certain times of the year, or involvement of body areas that cannot be exposed in public. In addition, potentially harmful UVA light is not blocked by this cream, and thus this approach may not be as safe as nbUVB phototherapy.
Summary
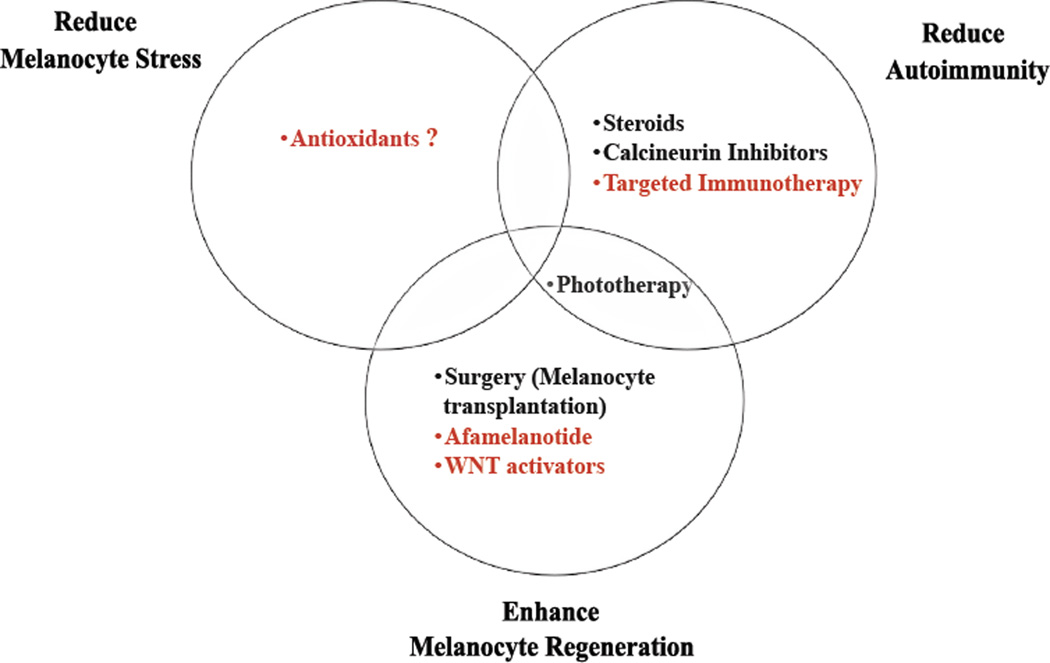
Research to better understand the pathogenesis of vitiligo has revealed that an optimal treatment strategy should consider 3 key aspects of the disease: 1) normalizing melanocyte stress, 2) inhibiting autoimmunity, and 3) promoting melanocyte regeneration. While current therapies such as phototherapy, topical immunomodulators, and surgical approaches partially address these conditions, they do so in a general, untargeted way, resulting in suboptimal responses and potential side effects. Emerging therapies seek to target specific pathways identified through basic, translational, and clinical research studies in vitiligo, in order to improve both efficacy and safety for patients (Fig. 3). While this is indeed a hopeful and exciting time for vitiligo patients and their physicians, this excitement should be balanced with caution, particularly since melanoma may take advantage of these same pathways to avoid immunosurveillance or promote their growth. However as with most medical treatments, careful patient selection and monitoring should enable us to normalize pathogenic responses in vitiligo to achieve homeostasis as in healthy individuals.
Fig. 3.
Current and emerging treatments address 3 major goals in vitiligo treatment. Current treatments are listed in black, emerging treatments in red.
Synopsis.
The pathogenesis of vitiligo involves interplay between intrinsic and extrinsic melanocyte defects, innate immune inflammation, and T cell-mediated melanocyte destruction. The goal of treatment is not only to halt disease progression, but promote repigmentation through melanocyte regeneration, proliferation, and migration. Thus treatment strategies that address all aspects of disease pathogenesis and repigmentation are likely to have greatest efficacy, a strategy that may ultimately require combination therapies. Current treatments generally involve non-targeted suppression of autoimmunity, while emerging treatments are likely to use a more targeted approach based on an in-depth understanding of disease pathogenesis, which may provide higher efficacy with a good safety profile.
Key Points.
Vitiligo results from the destruction of epidermal melanocytes by autoreactive cytotoxic T cells.
Melanocyte-specific autoimmunity in vitiligo is a result of interplay among multiple factors, including genetic predisposition, environmental triggers, melanocyte stress, and innate and adaptive immune responses.
Genome-wide association studies have identified multiple risk alleles in patients with vitiligo, and most are associated with immune regulation.
Melanocyte defects contribute to initiating autoimmunity in vitiligo.
Environmental stressors, especially chemical phenols that mimic the amino acid tyrosine and the products that contain them, can induce and exacerbate vitiligo.
An optimal treatment strategy in vitiligo would stabilize melanocytes, suppress the autoimmune response and restore immune tolerance, as well as stimulate melanocyte regeneration, proliferation, and migration to lesional skin.
A better understanding of the key pathways involved in vitiligo onset and progression will enable us to develop treatments that have greater efficacy and a good safety profile.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 2.Dell'Anna MLEK, Hamzavi I, Harris J, Parsad D, Taieb A, Picardo M. Vitiligo. Nature Reviews Disease Primers. 2015;1(1):1–16. doi: 10.1038/nrdp.2015.11. [DOI] [PubMed] [Google Scholar]
- 3.Rashighi M, Harris JE. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med. 2015;3(21):343. doi: 10.3978/j.issn.2305-5839.2015.11.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360(2):160–169. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- 5.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16(3):208–214. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 6.Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. doi: 10.1016/j.jaad.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44(6):676–680. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C, Gao J, Sheng Y, Dou J, Zhou F, Zheng X, et al. Genetic Susceptibility to Vitiligo: GWAS Approaches for Identifying Vitiligo Susceptibility Genes and Loci. Front Genet. 2016;7:3. doi: 10.3389/fgene.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J Invest Dermatol. 2012;132(2):268–273. doi: 10.1038/jid.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol Rev. 2016;269(1):11–25. doi: 10.1111/imr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boissy RE, Liu YY, Medrano EE, Nordlund JJ. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97(3):395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- 12.Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. The journal of investigative dermatology Symposium proceedings / the Society for Investigative Dermatology, Inc [and] European Society for Dermatological Research. 1999;4(1):91–96. doi: 10.1038/sj.jidsp.5640189. [DOI] [PubMed] [Google Scholar]
- 13.Shalbaf M, Gibbons NC, Wood JM, Maitland DJ, Rokos H, Elwary SM, et al. Presence of epidermal allantoin further supports oxidative stress in vitiligo. Exp Dermatol. 2008;17(9):761–770. doi: 10.1111/j.1600-0625.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 14.Koca R, Armutcu F, Altinyazar HC, Gurel A. Oxidant-antioxidant enzymes and lipid peroxidation in generalized vitiligo. Clin Exp Dermatol. 2004;29(4):406–409. doi: 10.1111/j.1365-2230.2004.01524.x. [DOI] [PubMed] [Google Scholar]
- 15.Dell'Anna ML, Ottaviani M, Albanesi V, Vidolin AP, Leone G, Ferraro C, et al. Membrane lipid alterations as a possible basis for melanocyte degeneration in vitiligo. J Invest Dermatol. 2007;127(5):1226–1233. doi: 10.1038/sj.jid.5700700. [DOI] [PubMed] [Google Scholar]
- 16.Schallreuter KU, Wood JM, Berger J. Low catalase levels in the epidermis of patients with vitiligo. J Invest Dermatol. 1991;97(6):1081–1085. doi: 10.1111/1523-1747.ep12492612. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons NC, Wood JM, Rokos H, Schallreuter KU. Computer simulation of native epidermal enzyme structures in the presence and absence of hydrogen peroxide (H2O2): potential and pitfalls. J Invest Dermatol. 2006;126(12):2576–2582. doi: 10.1038/sj.jid.5700612. [DOI] [PubMed] [Google Scholar]
- 18.Oliver E, Schwartz L, Warren L. Occupational leukoderma preliminary report. JAMA. 1939;113:927–928. [Google Scholar]
- 19.Fisher AA. Differential diagnosis of idiopathic vitiligo. Part III: Occupational leukoderma. Cutis. 1994;53(6):278–280. [PubMed] [Google Scholar]
- 20.Wu S, Li WQ, Cho E, Harris JE, Speizer F, Qureshi AA. Use of permanent hair dyes and risk of vitiligo in women. Pigment Cell Melanoma Res. 2015;28(6):744–746. doi: 10.1111/pcmr.12402. [DOI] [PubMed] [Google Scholar]
- 21.d'Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S, et al. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015;28(5):520–544. doi: 10.1111/pcmr.12393. [DOI] [PubMed] [Google Scholar]
- 22.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132(11):2601–2609. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Boorn JG, Picavet DI, van Swieten PF, van Veen HA, Konijnenberg D, van Veelen PA, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase haptenation and melanosome autophagy. J Invest Dermatol. 2011;131(6):1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 24.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124(4):798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Science translational medicine. 2013;5(174):174ra28. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levandowski CB, Mailloux CM, Ferrara TM, Gowan K, Ben S, Jin Y, et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc Natl Acad Sci U S A. 2013;110(8):2952–2956. doi: 10.1073/pnas.1222808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu R, Broady R, Huang Y, Wang Y, Yu J, Gao M, et al. Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin. PLoS One. 2012;7(12):e51040. doi: 10.1371/journal.pone.0051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Boorn JG, Jakobs C, Hagen C, Renn M, Luiten RM, Melief CJ, et al. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity. 2016;44(6):1406–1421. doi: 10.1016/j.immuni.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Current opinion in immunology. 2013;25(6):676–682. doi: 10.1016/j.coi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180(6):4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- 31.Mosenson JA, Eby JM, Hernandez C, Le Poole IC. A central role for inducible heat-shock protein 70 in autoimmune vitiligo. Exp Dermatol. 2013;22(9):566–569. doi: 10.1111/exd.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129(9):2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 33.Rork JF, Rashighi M, Harris JE. Understanding autoimmunity of vitiligo and alopecia areata. Curr Opin Pediatr. 2016 doi: 10.1097/MOP.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Science translational medicine. 2014;6(223):223ra23. doi: 10.1126/scitranslmed.3007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional Analysis of Vitiligo Skin Reveals the Alteration of WNT Pathway: A Promising Target for Repigmenting Vitiligo Patients. J Invest Dermatol. 2015;135(12):3105–3114. doi: 10.1038/jid.2015.335. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Wang Q, Wu J, Jiang M, Chen L, Zhang C, et al. Increased Expression of CXCR3 and its Ligands in Vitiligo Patients and CXCL10 as a Potential Clinical Marker for Vitiligo. Br J Dermatol. 2016 doi: 10.1111/bjd.14416. [DOI] [PubMed] [Google Scholar]
- 37.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132(7):1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schallreuter KU, Wood JM, Lemke KR, Levenig C. Treatment of vitiligo with a topical application of pseudocatalase and calcium in combination with short-term UVB exposure: a case study on 33 patients. Dermatology. 1995;190(3):223–229. doi: 10.1159/000246690. [DOI] [PubMed] [Google Scholar]
- 39.Schallreuter KU, Moore J, Behrens-Williams S, Panske A, Harari M. Rapid initiation of repigmentation in vitiligo with Dead Sea climatotherapy in combination with pseudocatalase (PC-KUS) Int J Dermatol. 2002;41(8):482–487. doi: 10.1046/j.1365-4362.2002.01463.x. [DOI] [PubMed] [Google Scholar]
- 40.Schallreuter KU, Kruger C, Wurfel BA, Panske A, Wood JM. From basic research to the bedside: efficacy of topical treatment with pseudocatalase PC-KUS in 71 children with vitiligo. Int J Dermatol. 2008;47(7):743–753. doi: 10.1111/j.1365-4632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- 41.Patel DC, Evans AV, Hawk JL. Topical pseudocatalase mousse and narrowband UVB phototherapy is not effective for vitiligo: an open, single-centre study. Clin Exp Dermatol. 2002;27(8):641–644. doi: 10.1046/j.1365-2230.2002.01142.x. [DOI] [PubMed] [Google Scholar]
- 42.Bakis-Petsoglou S, Le Guay JL, Wittal R. A randomized, double-blinded, placebo-controlled trial of pseudocatalase cream and narrowband ultraviolet B in the treatment of vitiligo. Br J Dermatol. 2009;161(4):910–917. doi: 10.1111/j.1365-2133.2009.09252.x. [DOI] [PubMed] [Google Scholar]
- 43.Gawkrodger DJ. Pseudocatalase and narrowband ultraviolet B for vitiligo: clearing the picture. Br J Dermatol. 2009;161(4):721–722. doi: 10.1111/j.1365-2133.2009.09292.x. [DOI] [PubMed] [Google Scholar]
- 44.Cohen BE, Elbuluk N, Mu EW, Orlow SJ. Alternative Systemic Treatments for Vitiligo: A Review. Am J Clin Dermatol. 2015;16(6):463–474. doi: 10.1007/s40257-015-0153-5. [DOI] [PubMed] [Google Scholar]
- 45.Szczurko O, Shear N, Taddio A, Boon H. Ginkgo biloba for the treatment of vitilgo vulgaris: an open label pilot clinical trial. BMC Complement Altern Med. 2011;11:21. doi: 10.1186/1472-6882-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28(3):285–287. doi: 10.1046/j.1365-2230.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 47.Middelkamp-Hup MA, Bos JD, Rius-Diaz F, Gonzalez S, Westerhof W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007;21(7):942–950. doi: 10.1111/j.1468-3083.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 48.Dell'Anna ML, Mastrofrancesco A, Sala R, Venturini M, Ottaviani M, Vidolin AP, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32(6):631–636. doi: 10.1111/j.1365-2230.2007.02514.x. [DOI] [PubMed] [Google Scholar]
- 49.Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012;25(1):88–98. doi: 10.1111/j.1755-148X.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villarino AV, Kanno Y, Ferdinand JR, O'Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194(1):21–27. doi: 10.4049/jimmunol.1401867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015;151(10):1110–1112. doi: 10.1001/jamadermatol.2015.1520. [DOI] [PubMed] [Google Scholar]
- 52.Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat Rev Drug Discov. 2012;11(2):103–104. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- 53.Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) J Am Acad Dermatol. 2016;74(2):370–371. doi: 10.1016/j.jaad.2015.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) J Am Acad Dermatol. 2015 doi: 10.1016/j.jaad.2015.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galli S, McLornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf. 2014;13(7):967–976. doi: 10.1517/14740338.2014.916273. [DOI] [PubMed] [Google Scholar]
- 57.Craiglow BG, Tavares D, King BA. Topical Ruxolitinib for the Treatment of Alopecia Universalis. JAMA Dermatol. 2016;152(4):490–491. doi: 10.1001/jamadermatol.2015.4445. [DOI] [PubMed] [Google Scholar]
- 58.Tufts Medical Center. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2000- [cited 2016 June 29] ]. Topical Ruxolitinib for the Treatment of Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT02809976 NLM Identifier: NCT02809976. [Google Scholar]
- 59.Zhao Y, Gartner U, Smith FJ, McLean WH. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. J Invest Dermatol. 2011;131(5):1045–1052. doi: 10.1038/jid.2011.41. [DOI] [PubMed] [Google Scholar]
- 60.Noel M, Gagne C, Bergeron J, Jobin J, Poirier P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004;3:7. doi: 10.1186/1476-511X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agarwal P, Rashighi M, Essien KI, Richmond JM, Randall L, Pazoki-Toroudi H, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135(4):1080–1088. doi: 10.1038/jid.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanderweil SG, Amano S, Ko W, Richmond J, Kelley M, Makredes Senna M, et al. A small double-blind, placebo-controlled, phase-II, proof-of-concept clinical trial to evaluate oral simvastatin as a treatment for vitiligo. J Am Acad Dermatol. 2016 doi: 10.1016/j.jaad.2016.06.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centre Hospitalier Universitaire de Nice. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2000- [cited 2016 Jan 19]]. Atorvastatin in Active Vitiligo . Available from: https://clinicaltrials.gov/ct2/show/NCT02432534 NLM Identifier: NCT02432534. [Google Scholar]
- 64.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72(2):221–236. doi: 10.1016/j.jaad.2014.07.033. quiz 37-8. [DOI] [PubMed] [Google Scholar]
- 66.RS, N VG. Targeting CTLA-4, PD-L1 and IDO to modulate immune responses in vitiligo. Exp Dermatol. 2016 doi: 10.1111/exd.13069. [DOI] [PubMed] [Google Scholar]
- 67.Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5(3):185–186. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 68.Brigham and Women's Hospital. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); [2000- [cited 2016 June 29]]. Open-label Pilot Study of Abatacept for the Treatment of Vitiligo. Available from: https://clinicaltrials.gov/ct2/show/NCT02281058 NLM Identifier: NCT02281058. [Google Scholar]
- 69. [[cited 2016 June 30]]; Genexin.com [Internet] Available from: http://www.genexine.com/m31.php. [Google Scholar]
- 70.Bulat V, Situm M, Dediol I, Ljubicic I, Bradic L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Coll Antropol. 2011;35(Suppl 2):147–151. [PubMed] [Google Scholar]
- 71.Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88(1):76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fabrikant J, Touloei K, Brown SM. A review and update on melanocyte stimulating hormone therapy: afamelanotide. J Drugs Dermatol. 2013;12(7):775–779. [PubMed] [Google Scholar]
- 73.Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, Lim HW. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013;149(1):68–73. doi: 10.1001/2013.jamadermatol.386. [DOI] [PubMed] [Google Scholar]
- 74.Lim HW, Grimes PE, Agbai O, Hamzavi I, Henderson M, Haddican M, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol. 2015;151(1):42–50. doi: 10.1001/jamadermatol.2014.1875. [DOI] [PubMed] [Google Scholar]
- 75.Harris JE. Melanocyte Regeneration in Vitiligo Requires WNT beneath their Wings. J Invest Dermatol. 2015;135(12):2921–2923. doi: 10.1038/jid.2015.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goren A, Salafia A, McCoy J, Keene S, Lotti T. Novel topical cream delivers safe and effective sunlight therapy for vitiligo by selectively filtering damaging ultraviolet radiation. Dermatol Ther. 2014;27(4):195–197. doi: 10.1111/dth.12115. [DOI] [PubMed] [Google Scholar]