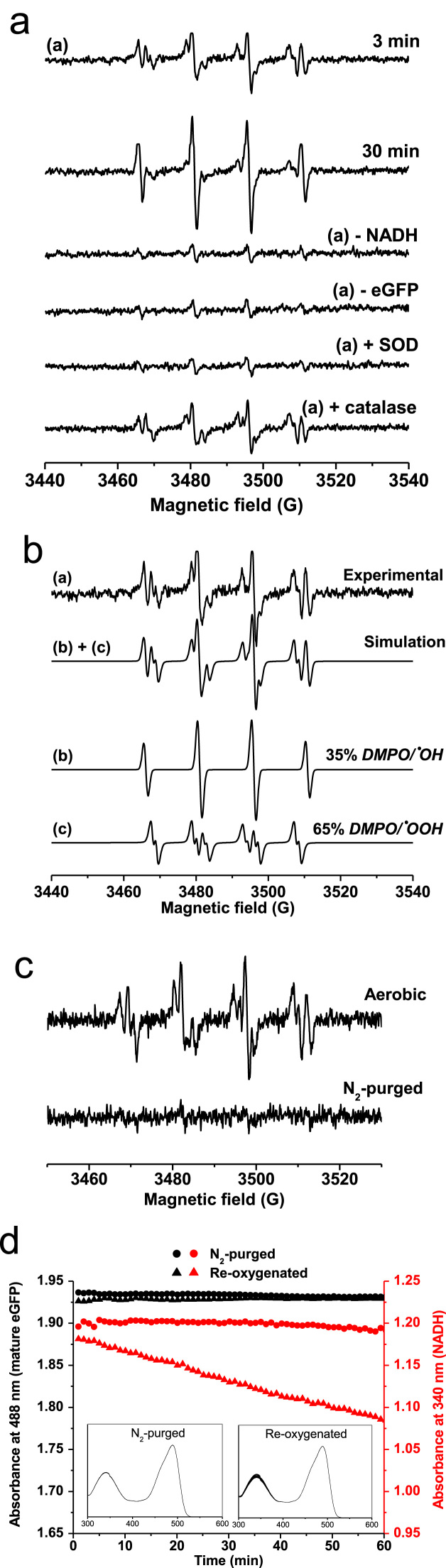
Fig. 1.
Reaction of NADH with eGFP leads to the formation of superoxide free radical anion. (a) Spin trapping of superoxide free radical anion (O2•–) generated by eGFP in the presence of NADH. Samples containing 50 μM GFP, 500 μM NADH and 100 mM DMPO showed a multiline-signal, trace (1). The effect of incubation time and eGFP or NADH was also studied. SOD was added to samples at 500 U/mL and catalase at 1 kU/mL. (b) Spectral simulation of trace (1) from panel A. The signal (1) is a superposition of (a) 65% DMPO/•OOH and (b) 35% DMPO/•OH. (c) ESR spin trapping for O2•– was prepared with samples containing 50 μM eGFP, 500 μM NADH and 100 mM DMPO using air-equilibrated solutions (aerobic) or anaerobic solutions (nitrogen-purged solutions). (d) A sample containing 25 μM eGFP was prepared without oxygen and tested for the ability to consume NADH at 250 μM (sample purged with nitrogen, •). This sample was then aerated with atmospheric air, and NADH consumption was followed (re-oxygenated sample, ▲). NADH was measured by its absorbance at 340 nm (ε340 nm=6220 M−1cm−1[33]). Black filled symbols were used to show the absorbance of the fluorescence-active, mature eGFP (• for the nitrogen-purged sample, and ■ for the sample after re-oxygenation, ε489 nm=55,000 M−1 cm−1).