Abstract
Introduction
The soluble urokinase-type plasminogen activator receptor (suPAR) has recently been associated with a decline in kidney function and incidence of chronic kidney disease in patients with cardiovascular disease undergoing cardiac catheterization, yet little is known whether suPAR is associated with deterioration of kidney function in the general population.
Methods
In the population-based Malmö Diet and Cancer Study cohort, plasma levels of suPAR were quantified in 5381 participants at baseline (1991–1994), and creatinine was measured and used to calculate estimated glomerulus filtration rate (eGFR) at baseline and follow-up (2007–2012). Incident chronic kidney disease was defined as eGFR < 60 ml/min per 1.73 m2 at follow-up.
Results
Participants within the highest quartile of suPAR had a significantly lower mean eGFR at follow-up than those within the lowest quartile (mean 68 vs. 74 ml/min per 1.73 m2; P-trend = 4.3 × 10–7). In multivariate regression analysis, suPAR (per 1 SD increment of log-transformed suPAR) was associated with a decline in eGFR (P = 3.3 × 10–9) and incident chronic kidney disease (561 events, odds ratio = 1.25; 95% confidence interval, 1.10–1.41). Furthermore, we identified 110 cases of hospitalization due to impaired kidney function via linkage to national registers of inpatient and outpatient hospital diagnoses. During a mean follow-up time of 19 years, suPAR was associated with risk for hospitalization due to impaired kidney function (hazard ratio = 1.49; 95% confidence interval, 1.27–1.74) in multivariate Cox proportional hazard analysis.
Discussion
The increased suPAR level at baseline was associated with a significantly higher longitudinal decline in eGFR, higher incidence of chronic kidney disease, and hospitalization due to impaired kidney function in a cohort of healthy middle-aged participants.
Keywords: chronic kidney disease, renal function, suPAR
Chronic kidney disease (CKD) is a growing public health issue, which today concerns around 8% to 16% of individuals worldwide, and a further disproportional increase is expected in developing countries.1 As the awareness of CKD in population is relatively low,2, 3 early detection of decreased kidney function is of major importance, not the least because CKD is associated with increased risk of cardiovascular events, hospitalization, and death.4 Creatinine and cystatin C are commonly used as markers for kidney function,5, 6 and to estimate the glomerular filtration rate (eGFR), which together with albuminuria is used to determine the stage of CKD.1 However, as both eGFR and albuminuria are relatively insensitive measures of early kidney injury, more sensitive biomarkers are needed to identify at-risk individuals earlier in the disease process to facilitate prevention of the progression to CKD.7
Very recently, elevated plasma levels of the soluble urokinase-type plasminogen activator receptor (suPAR) were shown to be strongly associated with an increased decline in kidney function and incidence of CKD in patients with cardiovascular disease (CVD) undergoing cardiac catheterization.8 Both suPAR and the membrane-bound form of the uPAR are known to be involved in the regulation of cell adherence and migration through binding of integrins,8, 9 and highly elevated suPAR levels have been implicated as potentially causal in the pathogenesis of focal segmental glomerulosclerosis through activation of β3 integrin that, when sufficiently activated, can lead to interference with podocyte migration and apoptosis.10, 11, 12, 13 In addition to kidney function, earlier studies have reported elevated suPAR levels in association with increased risk for several adverse health conditions, such as CVD,14, 15, 16 inflammation,17 and cancer.18
Earlier evidence hence indicates a role for elevated serum suPAR levels in focal segmental glomerulosclerosis and CKD in severely ill patients, but information about if circulating suPAR may play a role in kidney function of healthy individuals is lacking. Therefore, we challenged this question among generally healthy participants of the Malmö Diet and Cancer Study, a large population-based cohort from Southern Sweden, and aimed to investigate if circulating suPAR levels are associated with a longitudinal decline in kidney function, incidence of CKD, or hospitalization due to impaired kidney function, during a median follow-up time of more than 18 years.
Methods
Malmö Diet and Cancer Study
The Malmö Diet and Cancer Study (MDCS) is a population-based cohort study including men and women living in Malmö and born between 1923–1945 and 1923–1950. The participation rate was 40.8%.19 Written informed consent was given, and the MDCS was approved by the ethic committee at Lund University (LU 51-90). A detailed description of the cohort has been published elsewhere.20
For this study, we included individuals from the MDCS-Cardiovascular Cohort—a subcohort of the MDCS of 6103 randomly selected participants who underwent additional phenotyping, designed to study epidemiology of carotid artery disease, in between 1991 and 1994. Information on suPAR was available for 5381 individuals. We excluded participants with data missing for smoking (n = 137), fasting glucose (n = 20), body mass index (BMI) (n = 4), and creatinine (n = 85), leading to a final study population of, in total, 5135 participants.
Between 2007 and 2012, 3734 of those individuals who were alive and had not emigrated from Sweden (n = 4924) attended the follow-up re-examination, which has been described previously.21
Clinical Examination and Assays
During baseline examination, all participants underwent a physical examination and anthropometrics measurements were obtained by trained nurses. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured. BMI was calculated as weight/height2 (kg/m2). Questions concerning socioeconomic status, lifestyle factors, and medical history were assessed via a self-administered questionnaire.20 Fasting blood samples were drawn and immediately frozen to –80 °C and stored in a biological bank.22 Creatinine (μmol/l) was measured in plasma and analyzed with the Jaffé method, and traceable to the International Standardization with isotope dilution mass spectrometry. Cystatin C was measured using a particle-enhanced immunonephelometric assay (N Latex Cystatin; Dade Behring, Deerfield, IL). The values of cystatin C were not standardized because they were analyzed before the introduction of the world calibrator in 2010. The reference value for the method was 0.53 to 0.95 mg/l. The eGFR was calculated according to the previously reported CKD-Epidemiology Collaboration creatinine equation23 as follows: for males, eGFR = 141 × (Scr/0.9)α × 0.993age, where α is –0.411 if creatinine is ≤0.9 and –1.209 if creatinine is >0.9; for females, eGFR = 144 × (Scr/0.7)α × 0.993age, where α is –0.329 if creatinine is ≤0.7 and –1.209 if creatinine is >0.7. A factor of 0.0113 was included to convert creatinine levels measured in μmol/l into mg/dl. The concentration of suPAR (ng/ml) was analyzed in 2012 from frozen plasma blood samples, which were stored at –80 °C. A commercial ELISA suPARnostic® kit (Virogates, Copenhagen, Denmark) was used, which had an intraassay coefficient of variation of 3% and an interassay coefficient of variation of 5%. It has been shown previously that the stability of suPAR in plasma samples is high and it remains stable throughout several cycles of freezing and thawing.24
During the follow-up re-examination (2007–2012), anthropometric characteristics, and SBP and DBP were measured following similar approaches as at baseline. Furthermore, using the same analytical methods as at baseline, the plasma concentrations of glucose (mmol/l), creatinine (μmol/l), and cystatin C (mg/l) were measured in fasting blood samples.
Renal Outcomes
Data on eGFR at follow-up were available for 3193 participants, with measured baseline levels of suPAR; CKD at follow-up re-examination was defined as an eGFR of <60 ml/min per 1.73 m2. For the analysis of incident CKD, we excluded all participants with prevalent CKD at the baseline examination (n = 582).
In addition, we linked the MDCS cohort to the Swedish patient register to obtain information on hospitalization due to impaired kidney function. The Swedish patient register covers all hospitalizations in Sweden since 1987 and hospital outpatient visits from 2001 onward. The register has been previously described and validated for outcome classification.25 All participants were followed up until the occurrence of a hospital diagnosis of impaired kidney function, death, emigration from Sweden, or until 31 December 2013. In this study, we considered participants with admission to the hospital due to impaired kidney function defined as 585-586 according to International Classification of Diseases (ICD) 9, and N18 and N19 according to ICD10. We differentiated between impaired kidney function as the main diagnosis, registered as the first diagnosis in the patient record (110 cases), and impaired kidney function as the main or contributing diagnosis cases (patients with the above-mentioned ICD codes at any position, in total 207 cases). For the analysis of incident hospitalization due to impaired kidney function, we excluded all participants with prevalent impaired kidney function (n = 6).
Statistics
To reach normal distribution, we log-transformed suPAR levels and analyzed per 1 SD increment of the log value. In addition, the study sample was categorized into equal quartiles according to suPAR levels. We tested the association between suPAR and baseline characteristics using a general linear model (for continuous variables) adjusted for age and sex, and the χ2 test (for categorical variables). Furthermore, we tested the relationship between suPAR and eGFR at follow-up re-examination, for which we calculated the annual change in eGFR by dividing the variable “mean change over time” (value at follow-up minus value at baseline) by follow-up time in years to account for different lengths of follow-up.
Logistic regression was used to estimate the odds ratio and 95% confidence interval (CI) for incident CKD (eGFR at follow-up <60 ml/min per 1.73 m2) cases. For the basic model, we included age, sex, and follow-up time as covariates. We calculated the Net Reclassification Improvement (NRI)26 using the nri STATA command for the package idi from http://personalpages.manchester.ac.uk/staff/mark.lunt, and model discrimination was tested by calculating C-statistic using the roccomp command in STATA for models using risk factors with and without suPAR. Cox proportional hazard regression was used to estimate the hazard ratio (HR) and 95% CI for incident CKD cases. Age was used as the underlying time variable. Proportional hazard assumption was tested using Schoenfeld residuals (estat STATA command) and graphically (stphplot STATA command). The hazard function was graphically examined by plotting the Kaplan-Meier failure function (sts graph STATA command) according to the quartiles of suPAR.
In the basic model, we included age and sex as covariates. The final model for both Cox and logistic regression was adjusted for further risk factors of CKD: fasting glucose levels, eGFR, BMI, SBP, smoking status (current, former, or never smokers), and use of antihypertensive treatment (yes/no) at baseline.
A P value of ≤0.05 was considered to be statistically significant. SPSS (version 21, IBM Corporation, Armonk, NY) and STATA version 13 (StataCorp LP, College Station, Texas, USA) were used for analysis.
Results
Baseline Characteristics According to the suPAR Concentrations in the MDCS-Cardiovascular Cohort
Table 1 shows the baseline characteristics of the 5135 participants according to the quartiles of suPAR concentration at baseline. A positive association was observed between increased levels of suPAR with increased age, weight, BMI, SBP, DBP, waist, and glucose, and with female sex, antihypertensive treatment, and current smoking.
Table 1.
Clinical characteristicsa of the Malmö Diet and Cancer Study participants at baseline examination (1991–1996) according to the quartiles of suPAR
| N | All | Q1 | Q2 | Q3 | Q4 | P valueb | |
|---|---|---|---|---|---|---|---|
| Mean (range) suPAR (ng/ml) | 5135 | 3.0 (0.0–35.9) | 2.1 (0.0–2.4) | 2.6 (2.4–2.8) | 3.1 (2.8–3.4) | 4.4 (3.4–35.9) | <0.001 |
| Age (yr) | 5135 | 57.6 (5.9) | 55.7 (5.7) | 57.34 (5.9) | 58.7 (5.7) | 58.8 (5.9) | <0.001 |
| Male sex, n (%) | 5135 | 2114 (41.2%) | 642 (50.0%) | 551 (42.9%) | 450 (35.1%) | 471 (36.7%) | <0.001 |
| Height (cm) | 5135 | 169 (8.9) | 171 (9.04) | 169 (8.9) | 168 (8.83) | 168 (8.7) | 0.04 |
| Weight (kg) | 5135 | 74 (13.6) | 73 (13.09) | 73 (12.63) | 74 (13.87) | 74 (14.59) | <0.001 |
| BMI (kg/m2) | 5135 | 25.7 (3.9) | 25.1 (3.4) | 25.5 (3.6) | 26.0 (4.0) | 26.2 (4.6) | <0.001 |
| SBP (mm Hg) | 5135 | 142 (19.0) | 138 (17.2) | 141 (18.8) | 143 (19.6) | 144 (19.9) | <0.001 |
| DBP (mm Hg) | 5135 | 87 (9.3) | 86 (8.7) | 87 (9.5) | 87 (9.5) | 87 (9.7) | 0.028 |
| Waist (cm) | 5134 | 83.8 (12.9) | 83.2 (12.5) | 83.3 (12.5) | 83.7 (13.0) | 84.9 (13.6) | <0.001 |
| Fasting glucose (mmol/l) | 5135 | 5.2 (1.4) | 5.0 (0.8) | 5.1 (1.2) | 5.2 (1.4) | 5.4 (1.9) | <0.001 |
| Cystatin C (mg/l) | 4814 | 0.8 (0.2) | 0.7 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.9 (0.2) | <0.001 |
| P-Creatinine (μmol/l) | 5135 | 84.8 (16.4) | 85.3 (14.6) | 85.4 (14.4) | 83.6 (15.0) | 85.0 (20.5) | 0.829 |
| eGFR (ml/min per 1.73 m2) | 5135 | 76.0 (13.7) | 78.1 (13.3) | 75.7 (13.1) | 75.3 (13.4) | 75.1 (14.9) | 0.145 |
| AHT, n (%) | 5135 | 889 (17.8%) | 166 (12.9%) | 196 (15.3%) | 252 (19.6%) | 275 (21.4%) | <0.001 |
| Current smoking, n (%) | 5135 | 1377 (26.8%) | 192 (15.0%) | 226 (17.6%) | 316 (24.6%) | 643 (50.1%) | <0.001 |
AHT, antihypertensive treatment; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation23; SBP, systolic blood pressure; suPAR, soluble urokinase-type plasminogen activator receptor.
Data shown as mean ± SD or n (%).
P value adjusted for age and sex, or χ2 (1df) test (categorical variables).
suPAR Levels and Longitudinal Change in Kidney Function
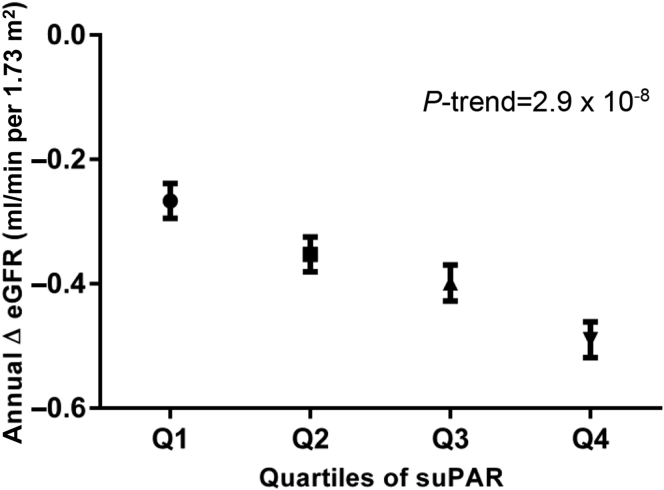
At follow-up re-examination, the mean eGFR was 70.15 (SD, 15.21) ml/min per 1.73 m2 in the 3193 participants. Compared with participants within the lowest quartile of suPAR, participants in the top quartile had a significantly lower eGFR (73.95 vs. 67.75 ml/min per 1.73 m2; P-trend = 4.3 × 10–7). Participants within the top quartile of baseline suPAR had a higher annual decline in eGFR than those within the lowest quartile of baseline suPAR (P-trend = 2.9 × 10–8) (Figure 1).
Figure 1.
Annual change in eGFR between baseline and follow-up re-examination in 3193 participants of the Malmö Diet and Cancer Study-Cardiovascular Cohort according to the quartiles of suPAR concentration at baseline. The general linear model was adjusted for age, sex, baseline levels of eGFR, and follow-up time. Concentration of suPAR at baseline Q1: 0.03–2.36, Q2: 2.36–2.73, Q3: 2.73–3.26, and Q4: 3.26–15.64. eGFR, estimated glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation23; suPAR, soluble urokinase-type plasminogen activator receptor.
suPAR and Incidence of CKD During Follow-up
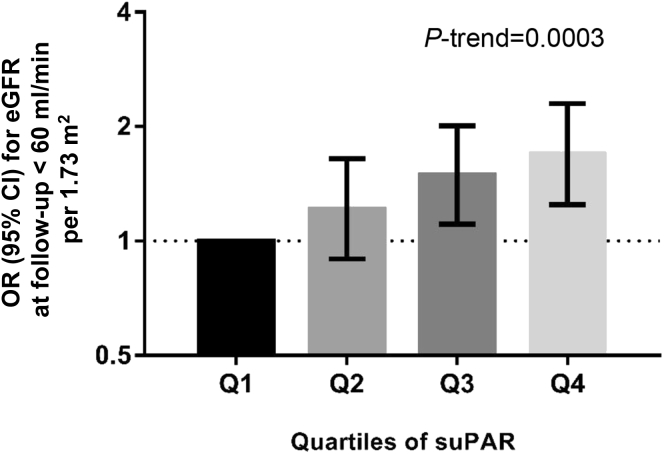
During a mean follow-up time of 16.6 (range, 13.3–20.2) years, incident CKD (eGFR < 60 ml/min per 1.73 m2 at follow-up re-examination) was present in 561 (231 men and 330 women) participants. In the basic model, the incidence of CKD was increased by 23% per 1 SD increase of log suPAR concentration at baseline (odds ratio, 1.23; 95% CI, 1.10–1.38). After additional adjustment for known risk factors (baseline levels of eGFR, fasting glucose, BMI, SBP, antihypertensive treatment, smoking), the risk increase remained similar (odds ratio, 1.25; 95% CI, 1.10–1.41). Compared with participants within the lowest quartile of suPAR, participants within the highest quartile had 69% increased incidence of CKD (Q4 odds ratio, 1.69; 95% CI, 1.25–2.30) (Figure 2).
Figure 2.
Risk for incident chronic kidney disease (eGFR ≤ 60 ml/min per 1.73 m2) at follow-up re-examination in 2851 participants of the Malmö Diet and Cancer Study-Cardiovascular Cohort according to the quartile of suPAR concentration at baseline. The logistic regression model was adjusted for age, sex, baseline levels of eGFR, fasting glucose, body mass index, systolic blood pressure, antihypertensive treatment (yes/no), smoking (current, ex, former), and follow-up time. Concentration of suPAR at baseline Q1: 0.03–2.35, Q2: 2.35–2.70, Q3: 2.71–3.25, and Q4: 3.25–15.64. N/cases: Q1: 712/94, Q2: 713/132, Q3: 713/163, and Q4: 713/172. CI, confidence interval; eGFR, estimated glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation23; OR, odds ratio; suPAR, soluble urokinase-type plasminogen activator receptor.
Risk Discrimination and NRI
We added suPAR to a model with conventional risk factors (i.e., sex and baseline age, eGFR, fasting glucose, SBP, antihypertensive medication, BMI, and smoking) and follow-up time to test the incremental value in discriminating between participants with and without CKD at follow-up re-examination. The area under the curve of the receiver operating characteristics was only marginally improved (area under the curve without suPAR 0.729 vs. area under the curve with suPAR 0.733, P = 0.17). However, adding suPAR to the risk model led to a significant NRI for 15.5% of the individuals (P = 0.0010). Model calibration was acceptable (Hosmer-Lemeshow’s P > 0.05) for both models with and without suPAR.
suPAR and Hospitalization due to Impaired Kidney Function
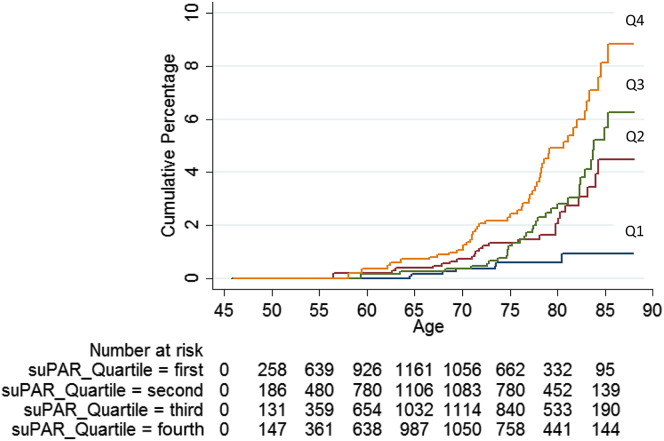
During a mean follow-up time of 19.04 (range, 0–22.25) years, 110 individuals (63 men and 47 women) were admitted to the hospital due to impaired kidney function as the main diagnosis. The event rate was 1.13 per 1000 person-years. The HR for incidence of impaired kidney function per 1 SD increase of log suPAR was 1.59 (95% CI, 1.41–1.79) in the basic model and remained significant in the fully adjusted model (HR = 1.49; 95% CI, 1.27–1.74). Hospitalization due to impaired renal function was significantly more common among participants within the highest quartile of baseline suPAR (Table 2). Adjusted for sex, participants within the highest suPAR quartile had an HR of 6.89 (95% CI, 3.11–15.28) compared with those within the lowest quartile of suPAR. After adjusting for further risk factors, the HR remained significantly increased (HR = 3.73; 95% CI, 1.65–8.44) (Figure 3).
Table 2.
Incidence of impaired kidney function (main diagnosis) during a mean follow-up time of 19 yr, in relation to baseline concentration of suPAR in the Malmö Diet and Cancer-Cardiovascular Cohort
| Per quartile of suPAR | Q1 | Q2 | Q3 | Q4 | Per 1 SD log suPAR | |
|---|---|---|---|---|---|---|
| Mean (range) suPAR (ng/ml) | 2.12 (0.03–2.43) | 2.62 (2.43–2.83) | 3.10 (2.83–3.41) | 4.36 (3.41–35.86) | ||
| Impaired kidney function as the main diagnosis | ||||||
| N/casesa | 5129/110 | 1282/7 | 1282/24 | 1283/32 | 1282/47 | |
| Sex-adjusted HR (95% CI) | 1.69 (1.40–2.04) | 1.0 | 3.17 (1.37–7.37) | 4.18 (1.84–9.50) | 6.89 (3.11–15.28) | 1.59 (1.41–1.79) |
| Risk factorb-adjusted HR (95% CI) | 1.37 (1.22–1.67) | 1.0 | 2.67 (1.15–6.22) | 3.11 (1.36–7.10) | 3.73 (1.65–8.44) | 1.49 (1.27–1.74) |
| All impaired kidney function cases | ||||||
| N/casesc | 5129/207 | 1282/23 | 1282/47 | 1283/57 | 1282/80 | |
| Sex-adjusted HR (95% CI) | 1.46 (1.28–1.67) | 1.0 | 1.84 (1.12–3.03) | 2.20 (1.35–3.59) | 3.50 (2.19–5.57) | 1.46 (1.32–1.61) |
| Risk factorb-adjusted HR (95% CI)c | 1.24 (1.08–1.43) | 1.0 | 1.55 (0.94–2.56) | 1.69 (1.04–2.76) | 2.13 (1.32–3.46) | 1.35 (1.18–1.53) |
AHT, antihypertensive treatment; BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation23; HR, hazard ratio; SBP, systolic blood pressure, suPAR, soluble urokinase-type plasminogen activator receptor.
Admission to the hospital due to impaired kidney function as main diagnosis.
Adjusted for sex, fasting glucose levels, eGFR, BMI, SBP, smoking status (current, former, or never smokers), and use of AHT (yes/no) at baseline. Age was used as an underlying time variable.
Admission to the hospital due to impaired kidney function as the main diagnosis (n = 110) and the secondary diagnosis (n = 97, in total n = 207).
Figure 3.
Cumulative incidence of hospitalization due to impaired renal function (main diagnosis, n = 110) during follow-up according to the quartiles of suPAR in 5129 participants in the Malmö Diet and Cancer Study-Cardiovascular Cohort. In the final model, male sex (HR: 2.53; 95% CI, 1.69–3.79), BMI (HR: 1.10; 95% CI, 1.05–1.15), baseline glucose (HR: 1.21; 95% CI, 1.14–1.30), AHT (HR: 1.59; 95% CI, 1.05–2.41), eGFR (HR: 0.96; 95% CI, 0.95–0.98), and current smoking (HR: 2.23; 95% CI, 1.34–3.73) were significantly associated with hospitalization due to impairment of renal function, in addition to suPAR. The Kaplan-Meier plot shows cumulative percentages of main cases of impaired renal function during follow-up in quartiles: first (lowest values) to fourth (highest values) quartile of the baseline suPAR concentration. Median (range) concentrations of the quartiles 1 to 4 are shown in Table 2. The numbers at risk are shown at 5-year intervals. Cox regression adjusted for sex, fasting glucose levels, eGFR, BMI, systolic blood pressure, smoking status (current, former, or never smokers), and use of antihypertensive treatment (AHT) (yes/no) at baseline. Age was used as an underlying time variable. BMI, body mass index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; suPAR, soluble urokinase-type plasminogen activator receptor.
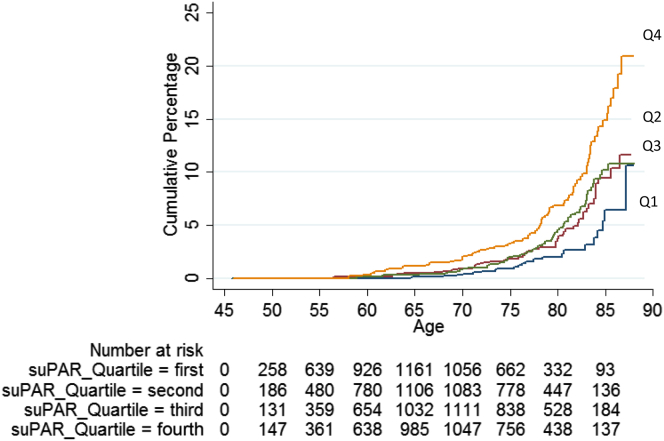
Similar results were observed when using a broader endpoint, including also individuals who were hospitalized with impairment of kidney function as a secondary diagnosis, which identified 97 additional cases, leading to 207 cases in total (128 men and 79 women) and an event rate of 2.12 per 1000 person-years (Table 2 and Figure 4).
Figure 4.
Cumulative incidence of hospitalization due to impaired renal function (all cases, n = 207) during follow-up according to the quartiles of suPAR in 5129 participants in the Malmö Diet and Cancer Study-Cardiovascular Cohort. The Kaplan-Meier plot shows cumulative percentages of all cases of impaired kidney function (n = 207) during follow-up in quartiles: first (lowest values) to fourth (highest values) quartile of the baseline suPAR concentration. Median (range) concentrations of the quartiles 1 to 4 are shown in Table 2. The numbers at risk are shown at 5-year intervals. Cox regression adjusted for sex, fasting glucose levels, estimated glomerular filtration rate, body mass index, systolic blood pressure, smoking status (current, former, or never smokers), and use of antihypertensive treatment (yes/no) at baseline. Age was used as underlying time variable. suPAR, soluble urokinase-type plasminogen activator receptor.
We tested the proportional hazard assumption of all Cox regression models, and the global P value for the Schoenfeld residuals in the final model for hospitalization due to impaired renal function as the main diagnosis was 0.099 and as the secondary diagnosis was 0.18, indicating no deviation from proportional hazard for the overall models. However, the assumption was violated by eGFR with a P value of 0.0010 and 0.0006, respectively. Therefore, because eGFR is known to decline by age, we formally tested interaction between age and eGFR on incidence of hospitalization due to impaired kidney function, and observed a strong interaction (P < 0.0001). However, adding such interaction term to the Cox models produced a similar estimated risk increase per 1 SD of log suPAR in the full model for hospitalization due to impaired kidney function as the main diagnosis, 1.51 (1.26–1.82) and as the secondary diagnosis, 1.37 (1.18–1.58), respectively.
Sensitivity Analyses
In sensitivity analyses, we excluded all cases classified as unspecified impaired kidney function (ICD9 586 or ICD10 N19) (n = 15) at first and last admission. HR estimates for main impaired kidney function (n = 109) were unchanged in the remaining 5114 participants (sex-adjusted HR = 1.59 [95% CI, 1.42–1.79] and fully adjusted HR = 1.49 [95% CI, 1.28–1.75] per increase in 1 SD log suPAR). Likewise, the estimates for all cases of impaired kidney function (in total n = 192) remained similar (sex-adjusted HR = 1.48 [95% CI, 1.34–1.64] and fully adjusted HR = 1.38 [95% CI, 1.21–1.57] per increase in 1 SD log suPAR).
In addition, we further excluded participants with prevalent diabetes or CVD at baseline (n = 325). Estimates remained unchanged (fully adjusted HR for impaired kidney function as the main diagnosis [n = 86] 1.45 [95% CI, 1.22–1.73] and all cases of impaired kidney function [n = 166] 1.32 [95% CI, 1.15–1.52] per 1 SD increment of suPAR, respectively).
Lastly, given the known association between baseline levels of suPAR and gender, we created sex-specific quartiles and tested the association between suPAR and hospitalization due to impaired kidney function. Hazard estimates remained similar (per increases in sex-specific quartile of suPAR main cases of impaired kidney function: sex-adjusted HR = 1.66 [95% CI, 1.37–2.01] and fully adjusted HR = 1.32 [95% CI, 1.09–1.62]; and all cases of impaired kidney function: sex-adjusted HR = 1.45 [95% CI, 1.26–1.65] and fully adjusted HR = 1.20 [95% CI, 1.05–1.39], respectively).
Discussion
The main findings from our population-based prospective cohort demonstrate that increased suPAR levels are associated with a decline in kidney function and hospitalization due to impaired kidney function in middle-aged men and women from southern Sweden, independently of traditional risk factors. It has recently been reported that elevated suPAR levels are associated with kidney function decline in patients with CVD undergoing cardiac catheterization,8 and that highly elevated suPAR may be causally linked to focal segmental glomerulosclerosis,10, 11, 12, 13 yet to our knowledge, our study is the first to show that suPAR predicts incidence of CKD and a longitudinal decline in kidney function in a generally healthy population. Thereby, our results are not only in line with the previously reported findings in severely ill patients,8 but also provide novel evidence for a broader application of suPAR as a predictive biomarker for declining kidney function.
Awareness is one of the key issues for individuals at risk for CKD or with CKD.27 Thus, early detection would be of immense importance as it may open opportunities for pharmacological and/or lifestyle-related preventive interventions.27, 28
In our study cohort, suPAR was not observed to add discriminative value on the top of the already established risk factors, as the C-statistics only marginally increased when suPAR was added to the model. Receiver operating characteristic is commonly used to evaluate how well a test or model can distinguish between a diseased and a nondiseased status (i.e., CKD in this study). One important aspect to keep in mind in regard to receiver operating characteristic analysis is that the effect on change in area under the curve depends on both the predictive ability of the “traditional risk model” and the strength of the new marker, and also on a potential correlation between them, and C-statistics may often be an insensitive measure.29, 30 Another aspect to consider is that suPAR levels may change over time, which in the scenario of a long follow-up most likely would reduce the observed associations between suPAR and CKD, and reduce the discrimination ability of rather insensitive measures like C-statistics. However, lack of information on suPAR levels at follow-up excludes such investigation in our study. Generally, the approach of reclassification is different, with the ability to determine how many individuals would be classified into the clinically relevant risk strata, thus directly comparing the clinical impact of 2 models.30 The results from our NRI analysis demonstrated that suPAR was able to significantly reclassify individuals into the correct risk direction. Hence, suPAR could be a potentially useful novel biomarker to identify individuals at high risk for developing CKD. Advantageously, we observed that elevated suPAR predicts future kidney dysfunction already a relatively long time before (on average >16 years) detection of declined eGFR at follow-up re-examination or hospitalization due to impaired kidney function.
Our results from the follow-up re-examination are supported by the additional analysis using data obtained from inpatient and outpatient registries on hospitalization due to impaired renal function (i.e., ICD9/10 585&586/N18&N19), which can be considered as a more strict definition, and also reflected in the lower number of cases in these analyses (110 cases with impaired renal function as the main diagnosis vs. 561 individuals classified with incident CKD at follow-up re-examination). We observed the expected effect modification between age and eGFR. However, as the estimates for suPAR remained unchanged after adding an interaction term (age × eGFR) to the used Cox models, the relationship between age and eGFR seemed not to markedly influence the interpretation of our results.
The clinical usability of novel biomarkers is of major importance as accurate early stratification of individuals at increased risk could enable targeting of preventive health resources. Previously, it has been shown that suPAR fulfills critical requirements for being a clinically usable biomarker of CKD in patients with CVD,8 considering that it has been shown to be stable in plasma,8, 31 and it is associated with incident CKD already in patients with still normal eGFR.8 Furthermore, suPAR has been shown to add prognostic value in patients with CVD and among subgroups with diabetes or hypertension,8 as well as in both whites and blacks regardless of the clear differences in prevalence of CKD between these ethnic groups.8 The close relation between heart and kidney disease makes our findings clinically useful in predicting risk of CKD in patients with CVD. In contrast to the study by Hayek et al.,8 our study population was generally healthy with a follow-up of more than 16 years, and expands the previous results, now highlighting its potential as an early risk marker also in the general population. In addition, the cNRI analysis demonstrates that suPAR could correctly reclassify individuals with respect to risk for future CKD, on top of other risk factors. Altogether, the results suggest that suPAR could be of relevance in a clinical setting.
suPAR has been suggested to have a specific role in the kidney, particularly in anchoring of podocytes in the basement membrane of the glomerulus through β3 integrin activation.12, 13 In line with earlier studies in severely ill patients,8 and in patients with focal segmental glomerulosclerosis,10, 11, 12, 13 our results now suggest that suPAR may also play a role in deterioration of kidney function in a generally healthy population. Therefore, more studies are needed to further explore the role of suPAR in kidney function in functional, preventive, and therapeutic settings.
Strengths and Limitations
In our study, we used kidney function data from several sources to investigate the role of circulating suPAR levels in a longitudinal decline of kidney function during a long follow-up with convincing results toward similar conclusions for all approaches. Yet, we need to note that all the participants of our study are Caucasians limiting the generalizability of our observations; however, the study by Hayek et al.8 reported that elevated suPAR was associated with a decline in kidney function similarly among Caucasians and Afro-American patients with CVD. Furthermore, it is important to note that all our main observations were detected independently of known risk factors, and that thorough sensitivity analyses (excluding either patients with unspecified impaired renal function, prevalent CVD, or diabetes, and using sex-specific tertiles of suPAR) provided similar estimates for hazard for hospitalization due to impaired renal function, demonstrating that most likely none of these significantly accounted for the observed associations.
Our study has some limitations that need to be discussed. Unfortunately, we do not have data for albuminuria in our cohort at baseline, which is a key limitation in the assessment of CKD stages 1 and 2. Furthermore, we have only measurements of cystatin C and creatinine at 2 time points, and more measurements would have been desirable as also recommended in the current KDIGO 2012 CKD guidelines.32 The Kidney Disease Improving Global Outcomes criteria for the classification of CKD require an eGFR of ≤60 ml/min per 1.73 m2 for a duration of >3 months; as there was only 1 follow-up visit, this was not possible to obtain. Yet, a similar definition for CKD was used by Hayek et al.8 Nevertheless, it needs to be pointed out that the long follow-up time of our study increases the confidence in assessing CKD progression.32 Furthermore, the results using the Swedish registry data on hospitalization due to impaired kidney function strongly supported the results observed based on changes in eGFR, not the least because this endpoint was assessed independently of the CKD definition at follow-up re-examination. Lastly, our study design provides evidence for the association between suPAR and kidney function decline, yet we cannot prove causality.
Conclusion
The findings of this prospective cohort study of generally healthy middle-aged participants from Sweden indicate that increased circulating suPAR level is associated with increased risk for the future decline of kidney function, and hospitalization due to impaired kidney function. Overall, the results of our study highlight circulating suPAR as a potential novel biomarker to identify individuals at increased risk for the decline in kidney function in the general population.
Disclosure
This study was supported by the Swedish Research Council, the Swedish Heart and Lung Foundation, the Novo Nordic Foundation, the Swedish Diabetes Foundation, and the Påhlsson Foundation, Skåne University Hospital, Ernhold Lundström, and by equipment grants from the Knut and Alice Wallenberg Foundation, the Region Skåne, Skåne University Hospital, Ernhold Lundström Foundation, the Linneus Foundation for the Lund University Diabetes Center, and the European Research Council (Consolidator grant number 649021, to MO-M).
Acknowledgments
We thank all participants of the Malmö Diet and Cancer Study.
References
- 1.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Plantinga L.C., Boulware L.E., Coresh J. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168:2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan Y., Zhang Q., Liu Z. Prevalence and risk factors associated with chronic kidney disease in adults over 40 years: a population study from Central China. Nephrology. 2010;15:354–361. doi: 10.1111/j.1440-1797.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 4.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Anderson S., Halter J.B., Hazzard W.R. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009;20:1199–1209. doi: 10.1681/ASN.2008080860. [DOI] [PubMed] [Google Scholar]
- 6.Stevens L.A., Coresh J., Schmid C.H. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassett R.G., Venuthurupalli S.K., Gobe G.C. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 8.Hayek S.S., Sever S., Ko Y.A. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thuno M., Macho B., Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei C., Moller C.C., Altintas M.M. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 11.Yoo T.H., Pedigo C.E., Guzman J. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol. 2015;26:133–147. doi: 10.1681/ASN.2013111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei C., El Hindi S., Li J. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei C., Trachtman H., Li J. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051–2059. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borne Y., Persson M., Melander O. Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur J Heart Fail. 2014;16:377–383. doi: 10.1002/ejhf.49. [DOI] [PubMed] [Google Scholar]
- 15.Edsfeldt A., Nitulescu M., Grufman H. Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke. 2012;43:3305–3312. doi: 10.1161/STROKEAHA.112.664094. [DOI] [PubMed] [Google Scholar]
- 16.Persson M., Ostling G., Smith G. Soluble urokinase plasminogen activator receptor: a risk factor for carotid plaque, stroke, and coronary artery disease. Stroke. 2014;45:18–23. doi: 10.1161/STROKEAHA.113.003305. [DOI] [PubMed] [Google Scholar]
- 17.Backes Y., van der Sluijs K.F., Mackie D.P. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38:1418–1428. doi: 10.1007/s00134-012-2613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bock C.E., Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13–39. doi: 10.1002/med.10054. [DOI] [PubMed] [Google Scholar]
- 19.Manjer J., Carlsson S., Elmstahl S. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10:489–499. doi: 10.1097/00008469-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Berglund G., Elmstahl S., Janzon L., Larsson S.A. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosvall M., Persson M., Ostling G. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: the Malmo Diet and Cancer Study. Atherosclerosis. 2015;239:615–621. doi: 10.1016/j.atherosclerosis.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Pero R.W., Olsson A., Berglund G. The Malmo biological bank. J Intern Med. 1993;233:63–67. doi: 10.1111/j.1365-2796.1993.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 23.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kofoed K., Schneider U.V., Scheel T. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52:1284–1293. doi: 10.1373/clinchem.2006.067595. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina M.J., D'Agostino R.B., Sr., D'Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [discussion: 207–212] [DOI] [PubMed] [Google Scholar]
- 27.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268:456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 28.Levey A.S., Eckardt K.U., Tsukamoto Y. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 29.Cook N.R. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 30.Cook N.R. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem. 2008;54:17–23. doi: 10.1373/clinchem.2007.096529. [DOI] [PubMed] [Google Scholar]
- 31.Andersen O., Eugen-Olsen J., Kofoed K. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80:209–216. doi: 10.1002/jmv.21114. [DOI] [PubMed] [Google Scholar]
- 32.Levin A., Stevens P.E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]