Figure 8. External Validation of AS Panel.
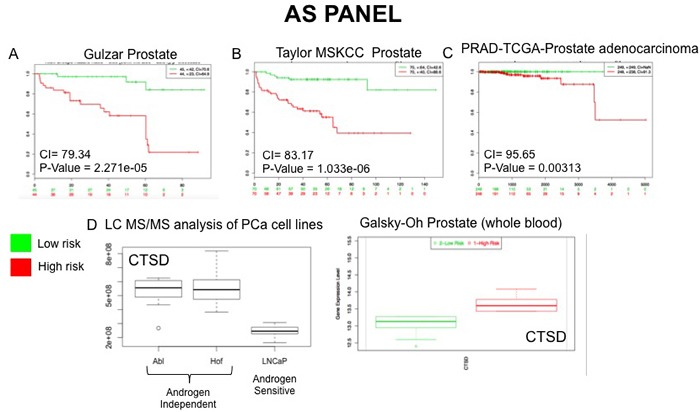
The SurvExpress bioinformatics resrouce was used to assess the potential clinical utility of proteins in the AS panel. Data from prostate cancer databases which contained data on the full panel of AS proteins was used to assess prognostic value of associated gene expression patterns between high and low risk PCa patients A.-C. Whole blood gene sequencing data from the Galsky-Oh database validated expression changes observed for the protein CTSD following unbiased LC-MS/MS analysis of PCa cell lines D.
