Figure 8. Schematic representation of how KLK6 protease could lead to degradation of intracellular α-synuclein aggregates.
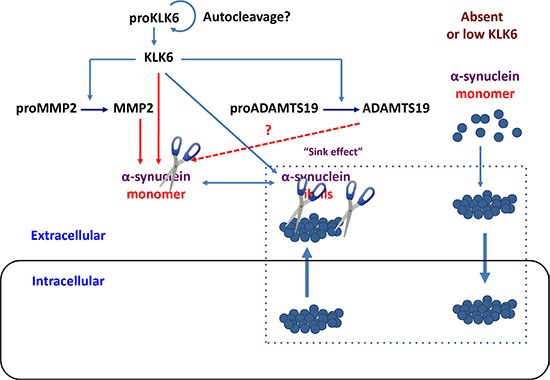
According to a hypothesis, known as sink effect (rectangular), intracellular and extracellular α-synuclein species are in equilibrium, thus, removal of extracellular α-synuclein could result in reduction of its intracellular levels with potential therapeutic applications. We propose that a proteolytic cascade is initiated by KLK6 that activates downstream metalloproteinases, such as MMP2 and ADAMTS19. The output result is the degradation of extracellular α-synuclein monomers and fibrils. Reduction of extracellular α-synuclein levels could lead to clearance of intracellular α-synuclein aggregates. Accumulation of α-synuclein, in the absence of KLK6 proteolysis could promote internalization of fibrilar species. This is supported by results shown in Figure 7, where Klk6–/– neurons have higher ability to uptake α-synuclein fibrils but not monomers. The α-synuclein monomers (dark blue circles) aggregate to yield fibrilar forms. Scissors depict proteolytic cleavage.
