Abstract
Background: Barth syndrome (BTHS) is a rare X-linked disorder that is characterized by mitochondrial abnormalities, cardio-skeletal myopathy, exercise intolerance, and premature mortality. The effect on endurance exercise training on exercise tolerance, cardio-skeletal function, and quality of life in BTHS is unknown.
Methods: Four young adults (23 ± 5 years, n = 4) with BTHS participated in a 12-week, supervised, individualized endurance exercise training program. Exercise training was performed on a cycle ergometer for 30–45′ three times per week at a moderate intensity level. Exercise tolerance was measured by graded exercise testing and peak oxygen consumption, heart function via two-dimensional and M-mode echocardiography, skeletal muscle function by near-infrared spectroscopy, and quality of life through the Minnesota Living with Heart Failure questionnaire.
Results: There were no adverse events during exercise testing or training for any participant. Peak oxygen consumption modestly (~5%) improved in three or four participants. Mean quality of life questions regarding dyspnea and side effects from medications significantly improved following exercise training. Mean resting heart function or skeletal muscle oxygen extraction during exercise did not improve after exercise training.
Conclusion: Endurance exercise training is safe and appears to modestly improve peak exercise tolerance and certain measures of quality of life in young adults with BTHS. However, compared to improvements resulting from endurance exercise training seen in other non-BTHS mitochondrial myopathies and heart failure, these improvements appear blunted. Further research into the most beneficial mode, intensity and frequency of exercise training in BTHS is warranted.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2016_553) contains supplementary material, which is available to authorized users.
Barth syndrome (BTHS) is an X-linked disorder that results in cardio-skeletal myopathy, heart failure, fatigue, chronic/cyclic neutropenia, growth delay, and premature mortality (Barth et al. 1983). The full scope of the pathological and clinical manifestations of BTHS is not fully understood, but involves a tafazzin gene defect that results in cardiolipin deficiency and severe mitochondrial dysfunction. BTHS-associated mitochondrial dysfunction is assumed to mediate the cardio-skeletal myopathy seen in the majority of those with BTHS (Spencer et al. 2006).
Our group recently found that whole-body peak oxygen consumption (VO2peak) during acute exercise was significantly impaired in BTHS when compared to healthy peers (Spencer et al. 2011). Further, deficits in VO2peak in those with BTHS were mediated by impairments in both cardiac and skeletal muscle function (Spencer et al. 2011). This is important because peak oxygen consumption (i.e., exercise tolerance) is the single best predictor of cardiovascular and all-cause mortality in individuals with cardiovascular disease (Myers et al. 2002). In individuals with non-BTHS heart failure, endurance (aerobic) exercise training increases peak whole-body oxygen consumption, left ventricular function, skeletal muscle blood flow and oxidative function, plasma lactate concentration during exercise, and improves serum markers associated with the severity of the cardiac impairment (e.g., tumor-necrosis factor-α (TNF-α) and pro-brain natriuretic peptide(pro-BNP)) (Sullivan et al. 1988; Minotti et al. 1990; Coats et al. 1992; Hambrecht et al. 1997, 1998; Adamopoulos et al. 2002; Delagardelle et al. 2002; Giannuzzi et al. 2003; Conraads et al. 2004; Bartlo 2007). Most importantly, endurance exercise training in patients with non-BTHS heart failure was safe, improved survival (Belardinelli et al. 1999), decreased hospitalization (Belardinelli et al. 1999), and increased quality of life (Coats et al. 1992; Tyni-Lenne et al. 1996; Belardinelli et al. 1999). Similarly, endurance exercise training has been found to be safe, improve exercise capacity, increase skeletal muscle oxygen extraction, and improve quality of life in individuals with other non-BTHS mitochondrial myopathies (Taivassalo et al. 2001; Cejudo et al. 2005; Jeppesen et al. 2006, 2009). However, the effect of endurance exercise training on these measures in BTHS is unknown. Because there is no specific therapies for BTHS to date, identification of an intervention to improve cardiovascular and metabolic health and to improve quality of life in this population is of high clinical importance.
BTHS has many similar clinical characteristics with patients with non-BTHS-related heart failure and those with non-BTHS mitochondrial myopathies. Therefore we hypothesized that endurance exercise training would improve exercise tolerance (i.e., peak oxygen consumption), skeletal muscle oxygen extraction, cardiac function, and quality of life in young adults with BTHS. In this pilot study, we collected preliminary data on the effect of a 12-week endurance exercise training program in four young adults with BTHS.
Methods
Participants
Males with BTHS ages 15–35 years were identified through the Barth Syndrome Foundation Registry (BSFR). Inclusion criteria included sedentary lifestyle (exercises <1×/week), willing to exercise, and with unchanged medications ≥3 months (Table 1). Participants were excluded if they had unstable heart disease or cardiac transplantation. Once identified, each potential participant was contacted by the principal investigator (WTC) to evaluate interest in participating in the study. If the participants were interested, a medical release for participation in the study’s exercise program was obtained from the participant’s personal cardiologist. Participant demographics are presented in Table 1. Informed written and verbal consent was obtained from all participants and the study was approved by the Human Research Protection Office at Washington University. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Table 1.
Demographics of participants
| Variable | Participant #1 | Participant #2 | Participant #3 | Participant #4 |
|---|---|---|---|---|
| Age (yrs) | 21 | 22 | 29 | 18 |
| ICD (yes/no) | Yes | Yes | Yes | No |
| Pre-intervention endurance (city blocks) | 2–3 | 2 | 2–3 | 1–2 |
| Employment/student status | Graduate student | Undergraduate student | Part-time employed | Unemployed |
| Hobbies | Following sports, cooking | Following sports, gaming | Computers, gaming, photography | Gaming |
| Medications | Carvedilol 25 mg BID | Metoprolol 50 mg BID | Carvedilol 12.5 mg BID | Losartan 25 mg QD |
| Furosemide 10 mg QD | Digoxin 0.125 mg QD | Captopril 25 mg QD | Atenolol 25 mg QD | |
| Digoxin 0.125 mg QD | Sertraline 50 mg QD | Carnitine 5 mL TID | ||
| Losartan 50 mg QD | Metformin 50 mg BID | CEQ-10 200 mg TID | ||
| Spironolactone 25 mg QD | Sulfamethoxazole TMP | |||
| Warfarin 2.5 mg QD | DS tablet QD | |||
| KCl 20 mg BID | ||||
| Arginine 7 g QD | ||||
| Cysteine 7 g QD | ||||
| Methionine 7 g | ||||
| Calcium/vitamin D 160 mg QD |
yrs years, ICD intra-cardiac defibrillator, CEQ co-enzyme Q, BID twice a day, QD once a day, TID three times a day
Baseline Testing
Baseline testing was performed at the 2010 Barth Syndrome International Family Scientific, Medical and Family Conference in Clearwater, FL. Upon screening, all participants received a physical examination, including a medical history. Participants also received fasting complete blood chemistry including plasma pro-brain natriuretic peptide as a marker of left ventricular (LV) dysfunction (Nir et al. 2004). All post-exercise measurements were performed at the Washington University Institute for Clinical and Translational Sciences Clinical Research Unit.
Exercise Testing
Each participant performed a graded exercise test on an electronically braked cycle ergometer (SensorMedics/VIASYS Healthcare, Yorba Linda, CA). The exercise test began with participants pedaling at a rate of 60 revolutions/min with no load for 1 min (warm up). After the warm-up period, the work rate (WR) on the cycle ergometer started at 10 Watts (W) and increased 10 W/min until volitional exhaustion. Twelve-lead cardiography (ECG), blood pressure, O2 consumption (VO2), CO2 production (VCO2), and ventilation (VMax, SensorMedics/VIASYS Healthcare) were continuously measured. Exercise tests were considered to be maximal if the peak heart rate (HR) was ≥85% of that predicted for age (220-age) and/or the peak respiratory exchange ratio (RER; VCO2/VO2) was ≥1.15 (ACSM 1998).
Echocardiography
All participants underwent echocardiography using a standardized protocol designed to evaluate LV size, LV morphology, and systolic and diastolic function as previously described (Spencer et al. 2011). All echocardiograms were recorded digitally and each study participant and visit was interpreted and measured by the same cardiologist (CLT) who was blinded to subject identification. Echocardiograms were obtained using Phillips 7500 echocardiography machines (Phillips Medical Systems, Bothell, WA).
Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is a well-described non-invasive technique that measures changes in oxy-hemoglobin and deoxy-hemoglobin which closely reflects muscular fractional oxygen extraction during exercise (DeLorey et al. 2003; Grassi et al. 2003). Relative concentration changes in oxy-(HbO2), deoxy-(DeoxyHb), and total (TotalHb) hemoglobin of the vastus lateralis muscle and brain were measured using a four-wavelength continuous wave NIRS system (FORE-SIGHT®, CAS Medical Systems Inc., Branford, CT) as previously described (Spencer et al. 2011).
Assessment of Heath-Related Quality of Life
Subjective quality of life was measured by the Minnesota Living with Heart Failure Questionnaire (MLWHFQ). This tool examines the effects of heart failure and treatments for heart failure on the individual’s quality of life and has been validated in individuals with heart failure (Rector et al. 1993) and a valid tool to evaluate therapies in this population (Rector et al. 1996). This tool contains 21 questions in response to the overall question “Did your heart failure prevent you from living as you wanted during the past month by?” in various categories (Supplementary Table 1) where the participants answer through a Likert scale from No (0) to Very Little (1) to Very Much (5).
Exercise Training
Volunteers participated in a 12-week, individualized, progressive, and supervised moderate-intensity endurance exercise training program performed at a hospital-based physical therapy clinic/cardiac rehabilitation program near the participants’ homes. The ultimate goal of the training program was for the participants to perform a total of 45 min of continuous or intermittent moderate-intensity exercise (Borg scale 14–16 or “Somewhat Hard to Hard”) on a cycle ergometer 3×/week or a total of 36 visits over 12 weeks. Cycle ergometer intensity (resistance in Watts) was increased to maintain moderate intensity as each participant’s exercise tolerance increased. The principal investigator (WTC) personally traveled to the training site to initiate and train the local physical therapist/exercise physiologist on the intervention protocol, based on the exercise tolerance of the participant. Weekly communication by the PI and site physical therapist/exercise physiologist was maintained throughout the training period. Total training time, heart rate, blood pressure, and perceived exertion (i.e., Borg Scale) were recorded for each training visit. In general, participants would exercise at a moderate intensity as long as they could and then rest before attempting another exercise bout. This was performed as many times within the 45 min time frame as the participant could tolerate with encouragement from the physical therapist/exercise physiologist.
Post-Exercise Testing
All post-exercise training testing was performed at Washington University School of Medicine as per previously described. Exercise testing and echocardiography equipment at Washington University were the same makes and models, and the NIRS equipment was identical as used in pre-testing at the 2010 Barth Syndrome Foundation Conference. Post-training NIRS was not performed in Participant #4 due to technical difficulties and thus data for this participant is not presented.
Statistics
The overall objective of this pilot study was to obtain preliminary data on the effect of endurance exercise training in BTHS. However, based on our preliminary data with peak exercise testing in BTHS (Spencer et al. 2011), for a statistically significant 20% improvement in peak oxygen consumption seen in populations with similar characteristics as BTHS, a sample size of 12 is needed. Pre–post data were analyzed through paired t-testing (IBM SPSS, Chicago, IL).
Results
Exercise Training
Participant #1 completed 100% of the exercise sessions plus three extra visits (39 visits) for a total of 1,920 min of exercise. Participant #2 completed 100% of the sessions (36 visits) for a total of 1,101 min of exercise. Participant #3 completed 100% of the exercise training plus eight visits extra (44 visits) due to personal reasons, there was a 10-day gap in the training where no exercise was performed and the extra visits were added to compensate for this. Participant #3 completed a total of 866 min of exercise. Participant #4 completed only 81% (29 sessions) of the exercise sessions for a total of 428 min of exercise (Table 2). Missed exercise sessions by Participant #4 were not due to medical but personal reasons. Also, compliance and motivation during the exercise sessions were reported to be variable in Participant #4 which might have affected the outcomes in this participant. Means ± SDs for heart rate, blood pressure, exercise duration, work and perceived exertion for each third of the training sessions (first third, second third, and final third) are presented in Table 3. Importantly, there were no adverse events for any participant during the training or testing sessions.
Table 2.
Endurance exercise training data
| Variable | Participant #1 | Participant #2 | Participant #3 | Participant #4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean +/−SD (session) | 1/3 | 2/3 | 3/3 | 1/3 | 2/3 | 3/3 | 1/3 | 2/3 | 3/3 | 1/3 | 2/3 | 3/3 |
| Exs duration (min) | 52 ± 9 | 49 ± 3 | 47 ± 3 | 14 ± 4 | 21 ± 3 | 41 ± 10 | 17 ± 1 | 22 ± 2 | 22 ± 3 | 16 ± 5 | 17 ± 9 | 12 ± 3 |
| HR (bpm) | 123 ± 7 | 123 ± 6 | 126 ± 2 | 136 ± 6 | 139 ± 6 | 145 ± 4 | 130 ± 2 | 131 ± 4 | 128 ± 4 | 153 ± 19 | 136 ± 8 | 145 ± 6 |
| SBP (mmHg) | 103 ± 6 | 107 ± 8 | 108 ± 7 | 114 ± 20 | 109 ± 12 | 98 ± 20 | 112 ± 10 | 109 ± 5 | 103 ± 9 | 102 ± 10 | 106 ± 9 | 109 ± 14 |
| DBP (mmHg) | 61 ± 6 | 60 ± 3 | 59 ± 4 | 66 ± 7 | 64 ± 7 | 77 ± 18 | 64 ± 6 | 68 ± 7 | 62 ± 4 | 60 ± 8 | 62 ± 5 | 61 ± 8 |
| Work (W) | 42 ± 10 | 42 ± 8 | 49 ± 4 | 15 ± 1 | 18 ± 2 | 21 ± 3 | 15 ± 0 | 15 ± 0 | 15 ± 1 | 25 ± 0 | 25 ± 0 | 100 ± 0 |
| RPE | 12 ± 1 | 14 ± 0 | 14 ± 1 | 13 ± 1 | 13 ± 0 | 13 ± 1 | 15 ± 1 | 16 ± 1 | 15 ± 1 | 15 ± 2 | 16 ± 2 | 16 ± 1 |
1/3 mean ± SD of first third of exercise sessions, 2/3 mean ± SD of second third of exercise sessions, 3/3 mean ± SD of last third of exercise sessions, Exs exercise, HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, RPE rating of perceived exertion, bpm beats per minute
Table 3.
Metabolic and exercise responses to endurance exercise training
| Variable | Participant #1 | Participant #2 | Participant #3 | Participant #4 | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean +/−SD | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | P value |
| Weight (kg) | 53.1 | 55.3 | 53.9 | 56.2 | 87.5 | 90.3 | 83.3 | 73.6 | 69.5 ± 18.5 | 68.9 ± 16.6 | 0.86 |
| Hb (g/dL) | 14.1 | 13.3 | 15.8 | 15.5 | 15.5 | 16.1 | 14.7 | 14.6 | 15.0 ± 0.8 | 14.9 ± 1.2 | 0.64 |
| Hct (%) | 40.6 | 37.1 | 44.3 | 44.3 | 44.0 | 46.4 | 43.1 | 41.5 | 43.0 ± 1.7 | 42.3 ± 4.0 | 0.62 |
| WBC (K/cumm) | 4.4 | 3.4 | 3.3 | 2.9 | 3.5 | 2.4 | 2.6 | 2.7 | 3.5 ± 0.7 | 2.9 ± 0.4 | 0.12 |
| ANC (K/cumm) | 1.6 | 1.1 | 0.5 | 0.6 | 1.7 | 0.7 | 0.7 | 0.4 | 1.1 ± 0.6 | 0.7 ± 0.3 | 0.16 |
| Neutrophil (%) | 37 | 33 | 13 | 22 | 49 | 31 | 25 | 13 | 31.0 ± 15.5 | 24.8 ± 9.2 | 0.36 |
| Pre-albumin (mg/dL) | 15.0 | 12.4 | 19.0 | 17.1 | 21.0 | 22.1 | 14.7 | 11.2 | 17.4 ± 3.1 | 15.7 ± 5.0 | 0.18 |
| BNP (pg/mL) | 196 | 121 | 26 | 43 | 15 | 18 | 10 | 15 | 61.8 ± 89.9 | 49.3 ± 49.5 | 0.60 |
| Peak VO2 (L/min) | 0.55 | 0.58 | 0.63 | 0.68 | 0.97 | 1.16 | 0.76 | 0.66 | 0.73 ± 0.18 | 0.77 ± 0.26 | 0.52 |
| Peak Work (W) | 50 | 60 | 50 | 60 | 80 | 70 | 70 | 60 | 62.5 ± 15.0 | 62.5 ± 5.0 | 1.00 |
| Peak Time (s) | 306 | 370 | 327 | 380 | 477 | 468 | 370 | 360 | 62.5 ± 15.0 | 62.5 ± 5.0 | 0.30 |
| Peak HR (bpm) | 144 | 151 | 142 | 169 | 171 | 169 | 171 | 136 | 157.0 ± 16.2 | 156.3 ± 16.0 | 0.96 |
| Peak RER | 1.9 | 1.7 | 1.7 | 1.7 | 1.4 | 1.3 | 1.7 | 1.4 | 1.7 ± 0.2 | 1.5 ± 0.2 | 0.10 |
| Peak Ve | 75 | 41 | 60 | 50 | 56 | 52 | ** | 30 | 63.7 ± 10.0 | 43.3 ± 10.0 | 0.22 |
| ΔDeoxy-Hb (μM/s) | 0.4 | 1.0 | 2.1 | 1.0 | 1.7 | 1.3 | ** | ** | 1.4 ± 0.9 | 1.1 ± 0.2 | 0.60 |
| SBP (mmHg) | 100 | 98 | 96 | 102 | 130 | 98 | 120 | 93 | 112 ± 16 | 113 ± 15 | 0.60 |
| DBP (mmHg) | 70 | 70 | 62 | 70 | 60 | 72 | 66 | 50 | 65 ± 4 | 66 ± 10 | 0.88 |
| LV mass index | 26.7 | 29.7 | 20.0 | 26.3 | 31.2 | 26.3 | 37.3 | 37.3 | 28.8 ± 7.3 | 29.9 ± 5.2 | 0.68 |
| LVEDV (mL) | 194.3 | 176.5 | 137.2 | 125.5 | 151.0 | 179.3 | 168.8 | 173.4 | 162.8 ± 24.6 | 162.5 ± 25.1 | 0.98 |
| LVESV (mL) | 157.0 | 125.6 | 72.4 | 69.1 | 79.5 | 100.7 | 79.6 | 78.7 | 97.1 ± 40.1 | 93.5 ± 25.1 | 0.76 |
| EF (%) | 19 | 29 | 47 | 45 | 47 | 44 | 53 | 55 | 42 ± 15 | 43 ± 11 | 0.67 |
| FS (%) | 9.0 | 8.9 | 19.4 | 19.1 | 20.3 | 19.8 | 26.9 | 26.6 | 18.9 ± 7.4 | 18.6 ± 7.3 | 0.96 |
| MPI | 0.9 | 0.7 | ** | ** | 0.44 | 0.54 | 0.51 | 0.68 | 0.62 ± 0.2 | 0.64 ± 0.1 | 0.86 |
| E/A | 2.7 | 1.8 | 1.6 | 1.5 | 1.4 | 1.4 | 1.5 | 1.4 | 1.8 ± 0.6 | 1.5 ± 0.2 | 0.28 |
Hb hemoglobin, Hct hematocrit, WBC white blood cell, ANC absolute neutrophil count, BNP brain natriuretic peptide, VO2 volume of oxygen consumption, Exs exercise, HR heart rate, RER respiratory exchange ratio, Ve ventilation, ΔDeoxy-Hb (μM/s) slope of deoxy-hemoglobin and time in micromoles per second, SBP systolic blood pressure, DBP diastolic blood pressure, LV left ventricle, LVEDV left ventricular end diastolic volume, LVESV left ventricular end systolic volume, FS fractional shortening, EF ejection fraction, MPI myocardial performance index, E/A early to late diastolic filling ratio
** Missing data
Demographics and Plasma Markers
As a group, there was no mean effect of exercise training on body weight; however, body weight slightly increased in three of the four participants and decreased in one participant. Mean hemoglobin/hematocrit, white cell count, absolute neutrophil count and percentage, pre-albumin, and pro-BNP were unchanged as a group following exercise training (Table 3).
Exercise Tolerance
As a group, mean peak oxygen consumption expressed absolutely and per kilogram body weight increased ~5% following exercise training; however, this was not significant (Table 3, Fig. 1a). Peak work, exercise time, and heart rate were unchanged following exercise training as a group. Peak respiratory exchange ratio (RER) tended (p = 0.10) to decrease following exercise training (Table 3). Three of four participants were able to tolerate more exercise volume (time exercising and intensity (watts)) from early in training to late in training (Fig. 1c).
Fig. 1.
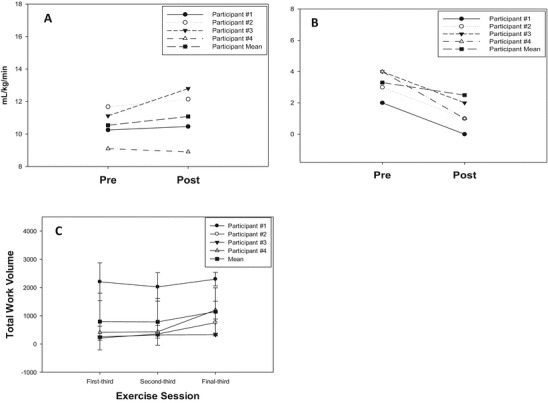
(a) Peak oxygen consumption before and following 12 weeks of endurance exercise. (b) Dyspnea scores from the Minnesota Living with Heart Failure Quality of Life Questionnaire before and following 12 weeks of endurance exercise. (c) Total exercise work volume (time × resistance) during over the first, second, and last third of exercise sessions
Muscle Oxygen Extraction
The mean slope of deoxy-hemoglobin and time (i.e., Δdeoxy-hemoglobin) during exercise did not change following exercise training (Table 3). The deoxy-hemoglobin slope improved in one participant but mildly declined in two participants (Table 3).
Cardiac Function
Mean resting heart rate, blood pressure, and systolic and diastolic function were unchanged as a group as a result of exercise training (Table 3).
Subjective Quality of Life
As a group, mean total score from the MLWHFQ did not improve following exercise training. However, the effect of the participants’ heart failure on the specific questions regarding dyspnea and side effects of medications significantly improved following exercise training (Fig. 1b, Supplementary Table 1). Of note, subjective fatigue improved in two participants and was unchanged in two participants after exercise training and the ability to perform house/yard work improved in two participants following training. However, as a group mean, neither specific question was significantly different following exercise training.
Discussion
This is the first study examine the effects of endurance exercise training on peak exercise tolerance, peak skeletal muscle oxygen extraction, cardiac function, and quality of life in individuals with BTHS. Our primary findings were that in four young adults with BTHS, 12 weeks of endurance exercise training: (1) was safe, (2) induced modest (~5%) improvement in peak exercise tolerance (i.e., oxygen consumption) in three of four participants, and (3) improved the participants’ subjective impact of heart failure on dyspnea and the side effects from treatment. In addition, the majority of participants were able to tolerate longer and more intense exercise as the training sessions increased. Surprisingly, endurance exercise training did not appear to improve skeletal muscle oxygen extraction or cardiac function to a significant extent these participants.
Overall, in four individuals with BTHS, endurance exercise training was safe as there were no adverse events or known occurrences of life-threatening arrhythmias. Of note, three of the four participants with BTHS had a previous history of life-threatening arrhythmias as evidenced by implantation of ICD’s but were able to safely undergo exercise training. These data suggest that individuals with BTHS with stable disease can participate in endurance-type exercise without risk of life-threatening arrhythmias. These data agree with the documented safety of aerobic exercise training in patients with non-BTHS congestive heart failure (Belardinelli et al. 1999). Additional benefits of endurance exercise training in non-BTHS heart failure include decreased hospitalization and improved survival rates (Belardinelli et al. 1999) however; additional and longer term studies are needed to confirm these benefits in BTHS.
Chronic endurance (i.e., aerobic) exercise training is a well-established intervention that improves exercise tolerance and increases cardiac function and skeletal muscle mitochondrial function in healthy individuals (ACSM 1998). Endurance exercise training has also been found to be safe and improve peak exercise tolerance and cardiac and skeletal muscle function in conditions that share similar characteristics as BTHS: mitochondrial myopathies (Cejudo et al. 2005; Jeppesen et al. 2006, 2009) and congestive heart failure (Coats et al. 1992; Delagardelle et al. 2002; Giannuzzi et al. 2003; Bartlo 2007). In patients with other mitochondrial myopathies, an endurance exercise program (3–5×/week, 12 weeks, 70% VO2peak) increased peak oxygen consumption 23–29% and peak work rate 16–100% (Cejudo et al. 2005; Jeppesen et al. 2006, 2009). Also, 14 weeks of endurance exercise training increased peak oxygen consumption (~25%), peak skeletal muscle oxygen extraction (20%), and mitochondrial function, enzyme activity and volume (~50%) without an improvement in cardiac function in individuals with mitochondrial myopathies. These findings demonstrate that endurance exercise training in non-BTHS mitochondrial myopathies specifically increases the ability of skeletal muscle to extract and utilize oxygen for energy production during exercise (Taivassalo et al. 2001). In addition, endurance exercise training increased peak oxygen consumption (23%) (Sullivan et al. 1988) and improved skeletal muscle oxidative function in individuals with non-BTHS heart failure (Sullivan et al. 1988; Minotti et al. 1990; Hambrecht et al. 1997). In the current study, young men with BTHS were able to tolerate greater amounts (i.e., time and resistance) of submaximal exercise as the training period progressed; however, we observed only a modest increase in peak oxygen consumption (~5%) compared to larger increases seen in other mitochondrial myopathies (20–30%). This might suggest an improved ability for Cori cycling/gluconeogenesis in BTHS with exercise training (Bongaerts and Wagener 2007); however, this is speculative as we did not measure this. We also did not find an improvement in skeletal muscle oxygen extraction during peak exercise as seen in studies in patients with non-BTHS mitochondrial myopathies. One potential reason for these differences might include slight differences in exercise intensity: we used perceived exertion (i.e., Borg scale) to guide exercise intensity rather than %VO2peak as used in other studies and thus we might have exercised our participants at a lower intensity level. Also, with the heterogeneity of mitochondrial DNA mutations, it is possible that exercise training increases the density of non-mutated mitochondria, resulting in better improvements in peak oxygen consumption. In contrast, the mitochondrial pathophysiology of BTHS is of nuclear origin and therefore endurance exercise training might only increase the density of defective mitochondria, thus blunting the response to exercise training. However, this is speculative as we did not obtain muscle biopsies. Lastly, it is possible that the training period (i.e., 12 weeks) was not long enough to elicit skeletal muscle and cardiac adaptations in the participants with BTHS. In healthy individuals, 12 weeks of endurance exercise is associated with a ~8–11% increase in peak oxygen consumption; however, improvements increase to 10–14%, 13–17%, and 16–17% with 24, 36, and 52 weeks of training, respectively (Iwasaki et al. 2003; Scharhag-Rosenberger et al. 2009). Thus, it is possible that cardiorespiratory adaptations might have further increased with a longer duration of exercise training and warrants further study.
Moderate-intensity endurance exercise training has been shown to improve left ventricular function in individuals with non-BTHS heart failure as well as improve levels of plasma markers known to be associated with the severity of cardiac impairment (e.g., TNF-α and pro-BNP) (Adamopoulos et al. 2002; Conraads et al. 2004; Chen et al. 2012). However, in the current study we did not observe improvements in resting nor exercise-stimulated left ventricular function, plasma pro-BNP, or plasma TNF-α following endurance exercise training in four participants with BTHS. It is possible that the training period was not long enough to demonstrate improvements in cardiac function as the majority of studies in non-BTHS heart failure that demonstrated cardiac improvements had training period ≥6 months (Chen et al. 2012). Like skeletal muscle, it is also possible that endurance exercise training in BTHS increased more genetically impaired cardiac mitochondria that did not result in improvement of cardiac energetics and function; however, this is also speculative as cardiac biopsies were not performed.
Endurance exercise training has been shown to improve subjective quality of life in non-BTHS heart failure (Tyni-Lenne et al. 1996; Belardinelli et al. 1999; Taylor et al. 2014) and mitochondrial myopathy (Cejudo et al. 2005). In the current study, endurance exercise training did not significantly improve overall (i.e., total score of the Minnesota Living with Heart Failure Questionnaire) subjective quality of life but did significantly improve scores on specific questions related to dyspnea and side effects of heart failure treatment. Two of four participants also reported an improvement in fatigue and rest during the day questions however as a group, these were not significantly improved following exercise training. Larger and longer studies are needed to fully determine the beneficial effects of endurance exercise training on subjective quality of life in BTHS.
There are limitations associated with this small pilot study. First, we were not adequately powered to demonstrate an effect of endurance exercise training on exercise tolerance. We were also not powered to detect differences in cardiac function or quality of life. However, we were able to show a trend towards improvement of exercise tolerance in the three of four participants with BTHS. In addition, during the training, exercise intensity was guided by perceived exertion rather than heart rate/VO2. This was performed as all participants were on beta-blocker medication which blunts exercise-stimulated heart rate thus making heart rate guided exercise prescription unreliable. Lastly, the endurance exercise intervention was applied by four different physical therapists/exercise physiologists that could have led to varying motivational techniques for each participant. However, the PI (WTC) provided the same instructions and general communication to all participating therapists/physiologists.
In conclusion, in four young adults with BTHS, 12 weeks of moderate-intensity endurance exercise training was safe, well-tolerated, and modestly improved exercise tolerance and specific areas of subjective quality of life. Training improvements in BTHS however were not as great as those seen in other conditions that share characteristics as BTHS. Endurance exercise induced adaptation might be blunted in BTHS due to the more severe cardiac involvement and homogenous mitochondrial alterations in BTHS compared to other mitochondrial myopathies (Spencer et al. 2006; Finsterer and Kothari 2014). Randomized clinical trials that examine higher intensity and longer duration interventions of endurance exercise training are needed to determine if endurance exercise training is clearly beneficial in BTHS. It is also possible that an exercise training mode that targets less oxidative muscle fiber types (i.e., Type II) such as resistance training (or combination of resistance and endurance) might be more effective in improving exercise tolerance in BTHS however; this needs to be tested in future studies.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Compliance with Ethics Guidelines
Synopsis
Conflict of Interest
Endurance exercise training is safe and appears to modestly improve exercise tolerance and certain measures of quality of life in young adults with BTHS.
W. Todd Cade, Dominic Reeds, Linda Peterson, Kathryn Bohnert, Rachel Tinius, Paul Benni, Barry Byrne, and Carolyn Taylor declare that they have no conflict of interest.
Author Contributions
WTC planned the study, performed the study, analyzed the data, wrote the manuscript.
DNR performed the studies, wrote the manuscript.
LRP performed the studies, wrote the manuscript.
KLB performed the studies, wrote the manuscript.
RAT performed the studies, wrote the manuscript.
PBB analyzed the data, wrote the manuscript.
BJB planned the study, wrote the manuscript.
CLT planned the study, analyzed the data, wrote the manuscript.
Funding
This project was supported by the Barth Syndrome Foundation and by the National Institutes of Health grants: Institute of Clinical and Translational Sciences (UL1 RR024992), Diabetes Research and Training Center (DK-020579), and Nutrition-Obesity Research Center (DK-056341) from the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH or its Institutes.
Footnotes
Competing interests: None declared
Contributor Information
W. Todd Cade, Email: tcade@wustl.edu.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:653–663. doi: 10.1016/S0735-1097(01)01795-8. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- Barth PG, Scholte HR, Berden JA, et al. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci. 1983;62:327–355. doi: 10.1016/0022-510X(83)90209-5. [DOI] [PubMed] [Google Scholar]
- Bartlo P. Evidence-based application of aerobic and resistance training in patients with congestive heart failure. J Cardiopulm Rehabil Prev. 2007;27:368–375. doi: 10.1097/01.HCR.0000300263.07764.4a. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.CIR.99.9.1173. [DOI] [PubMed] [Google Scholar]
- Bongaerts GP, Wagener DJ. Increased hepatic gluconeogenesis: the secret of Lance Armstrong’s success. Med Hypotheses. 2007;68:9–11. doi: 10.1016/j.mehy.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Cejudo P, Bautista J, Montemayor T, et al. Exercise training in mitochondrial myopathy: a randomized controlled trial. Muscle Nerve. 2005;32:342–350. doi: 10.1002/mus.20368. [DOI] [PubMed] [Google Scholar]
- Chen YM, Li ZB, Zhu M, Cao YM. Effects of exercise training on left ventricular remodelling in heart failure patients: an updated meta-analysis of randomised controlled trials. Int J Clin Pract. 2012;66:782–791. doi: 10.1111/j.1742-1241.2012.02942.x. [DOI] [PubMed] [Google Scholar]
- Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–2131. doi: 10.1161/01.CIR.85.6.2119. [DOI] [PubMed] [Google Scholar]
- Conraads VM, Beckers P, Vaes J, et al. Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. Eur Heart J. 2004;25:1797–1805. doi: 10.1016/j.ehj.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Delagardelle C, Feiereisen P, Autier P, Shita R, Krecke R, Beissel J. Strength/endurance training versus endurance training in congestive heart failure. Med Sci Sports Exerc. 2002;34:1868–1872. doi: 10.1097/00005768-200212000-00002. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol. 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- Finsterer J, Kothari S. Cardiac manifestations of primary mitochondrial disorders. Int J Cardiol. 2014;177:754–763. doi: 10.1016/j.ijcard.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Giannuzzi P, Temporelli PL, Corra U, Tavazzi L. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) Trial. Circulation. 2003;108:554–559. doi: 10.1161/01.CIR.0000081780.38477.FA. [DOI] [PubMed] [Google Scholar]
- Grassi B, Pogliaghi S, Rampichini S, et al. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol. 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Fiehn E, Yu J, et al. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29:1067–1073. doi: 10.1016/S0735-1097(97)00015-6. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.CIR.98.24.2709. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose–response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol. 2003;95:1575–1583. doi: 10.1152/japplphysiol.00482.2003. [DOI] [PubMed] [Google Scholar]
- Jeppesen TD, Schwartz M, Olsen DB, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129:3402–3412. doi: 10.1093/brain/awl149. [DOI] [PubMed] [Google Scholar]
- Jeppesen TD, Duno M, Schwartz M, et al. Short- and long-term effects of endurance training in patients with mitochondrial myopathy. Eur J Neurol. 2009;16:1336–1339. doi: 10.1111/j.1468-1331.2009.02660.x. [DOI] [PubMed] [Google Scholar]
- Minotti JR, Johnson EC, Hudson TL, et al. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest. 1990;86:751–758. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Nir A, Bar-Oz B, Perles Z, Brooks R, Korach A, Rein AJ. N-terminal pro-B-type natriuretic peptide: reference plasma levels from birth to adolescence. Elevated levels at birth and in infants and children with heart diseases. Acta Paediatr. 2004;93:603–607. doi: 10.1111/j.1651-2227.2004.tb02984.x. [DOI] [PubMed] [Google Scholar]
- Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-W. [DOI] [PubMed] [Google Scholar]
- Rector TS, Bank AJ, Mullen KA, et al. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–2141. doi: 10.1161/01.CIR.93.12.2135. [DOI] [PubMed] [Google Scholar]
- Scharhag-Rosenberger F, Meyer T, Walitzek S, Kindermann W. Time course of changes in endurance capacity: a 1-yr training study. Med Sci Sports Exerc. 2009;41:1130–1137. doi: 10.1249/MSS.0b013e3181935a11. [DOI] [PubMed] [Google Scholar]
- Spencer CT, Bryant RM, Day J, et al. Cardiac and clinical phenotype in Barth syndrome. Pediatrics. 2006;118:e337–e346. doi: 10.1542/peds.2005-2667. [DOI] [PubMed] [Google Scholar]
- Spencer CT, Byrne BJ, Bryant RM, et al. Impaired cardiac reserve and severely diminished skeletal muscle O(2) utilization mediate exercise intolerance in Barth syndrome. Am J Physiol Heart Circ Physiol. 2011;301:H2122–H2129. doi: 10.1152/ajpheart.00479.2010. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78:506–515. doi: 10.1161/01.CIR.78.3.506. [DOI] [PubMed] [Google Scholar]
- Taivassalo T, Shoubridge EA, Chen J, et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol. 2001;50:133–141. doi: 10.1002/ana.1050. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev. 2014;4:CD003331. doi: 10.1002/14651858.CD003331.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyni-Lenne R, Gordon A, Sylven C. Improved quality of life in chronic heart failure patients following local endurance training with leg muscles. J Card Fail. 1996;2:111–117. doi: 10.1016/S1071-9164(96)80029-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


