Figure 1.
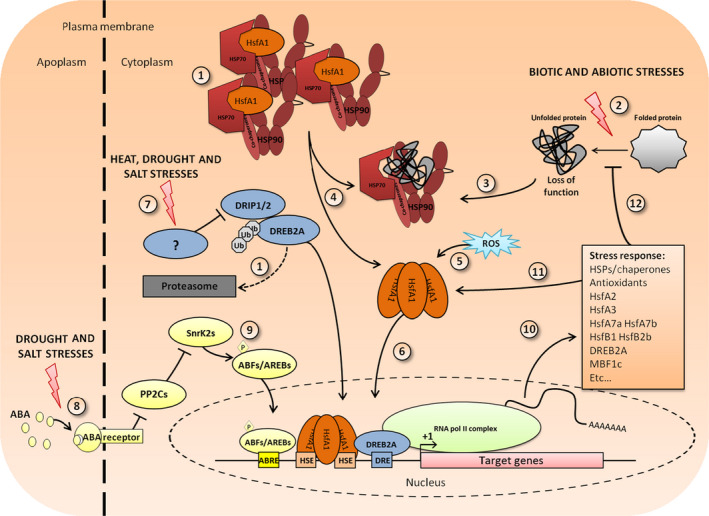
Schematic representation of the HSP/HSF pathway. (1) In nonstress conditions, class A1 HSFs are sequestered by HSP90/70 and their cochaperones and DREB2A is degraded through the UPS thanks to the E3 ligase DRIP1/2 (Qin et al., 2008). Upon stress application (2), the high number of misfolded proteins triggers the recruitment of HSP90/70 to its client and frees the HSFA1s following the chaperone titration model (4). In a high ROS context (5), the HSFA1s can form oligomers and are translocated in the nucleus (6) to bind HSE on DNA and induce target genes’ transcription. Trimers are represented here, in reference to mammalian HSF1 trimerization, even though the degree of oligomerization has not been established in plants except for AtHSFA1a trimerization. Other signalling pathways may interfere with the HSF/HSP pathway. Specific heat, drought and salinity stresses will lead to DRIP1/2 inhibition and DREB2A accumulation (7). Drought and salt stresses will induce ABA accumulation and binding to its receptor PYR/PYL/RCAR, leading to inactivation of PP2Cs (8). SnrK2s can then activate their target by phosphorylation (9). ABF/AREBs and DREB2A can then enter the nucleus, cooperatively or separately bind their target DNA motif, respectively, ABRE and DRE and HSE, to activate target genes’ expression (10). Induced proteins comprise stress‐specific ‘transcriptional relay’ TFs that feedback positively on HSF and HSP transcription (11) or proteins that participate in homeostasis re‐establishment (12). After the stress, the HSF/HSP content of the cell is different from the start. The quantity and the nature of the HSFs/HSP define the acclimated state.
