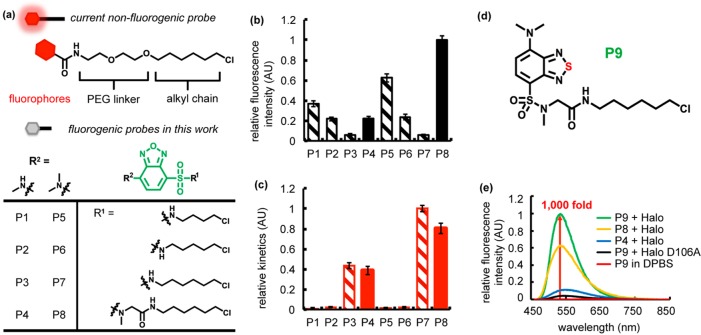
Figure 2.
Fluorogenic benzoxadiazole and benzothiadiazole probes for Halo tag. (a) Current nonfluorogenic probes (top) for Halo tag harbor a six-carbon alkyl chain, a polyethylene glycol (PEG) linker, and a fluorescent fluorophore. The table shows structures of fluorogenic probes for Halo tag evaluated in this work. R1 is a chloroalkane chain of varying length, with and without a sarcosine amide linker. R2 denotes the structure of an electron donating group, which is either a methylamino or dimethylamino substituent. (b) Relative intensity of conjugates of P1–P8 with Halo and (c) its relative labeling kinetics. Halo samples (20 μM) were incubated with P1–P8 (10 μM) for 18 h prior to fluorescence intensity measurements (Ex. = 450 nm, and Em. = 530 nm). The labeling rate was recorded upon mixing 5 μM Halo protein with 0.5 μM ligand (Ex. = 450 nm, and Em. = 530 nm). (d) P9 consists of a six-carbon alkyl chain, a sarcosine amide linker, and a solvatochromic fluorophore based on a benzothiadiazole scaffold. (e) P9 exhibits ∼1000-fold fluorescence enhancement upon reaction with Halo protein (green), compared to P9 in DPBS (red). The probe is weakly fluorescent upon binding to Halo-D106A (black). A solution of the protein (20 μM) was incubated with 0.5 equiv (10 μM) of P9 (green), P8 (yellow), or P4 (blue) in DPBS buffer at 25 °C for 1 h. Fluorescence emission spectra were recorded at 450 nm excitation.