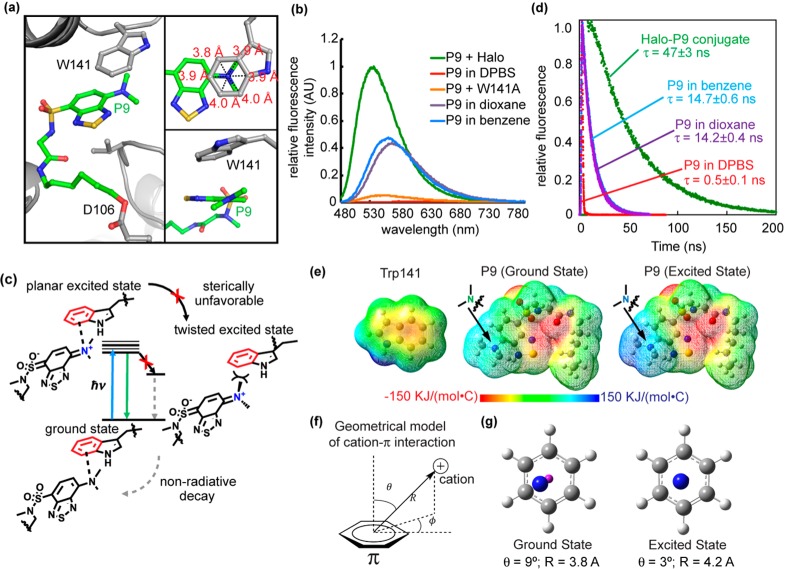
Figure 3.
Cation−π interaction enhances P9 fluorescence. (a) The benzothiadiazole ring of P9 is embedded inside Halo, accommodated by a shift of a loop containing Trp141. The inset at the top right shows that the tertiary N of the dimethylamino group resides close to the geometrical center of the benzene ring of the Trp141 indole (3.8–4.0 Å distance between N and benzene carbons). The inset at the bottom right shows that the dimethylamino group is oriented just slightly twisted in relative to the benzothiadiazole moiety. (b) Fluorescence spectra of the Halo–P9 conjugate (green), W141A–P9 conjugate (orange), P9 in DBPS buffer (red), P9 in 1,4-dioxane (purple), and P9 in benzene (blue). For the Halo–P9 and W141A–P9 conjugates, a solution of the protein (20 μM) was incubated with 0.5 equiv (10 μM) of P9 in DPBS buffer at 25 °C for 1 h. For P9 in different solvents, 10 μM P9 was added in solvents at 25 °C for 1 h. Fluorescence emission spectra were recorded at 450 nm excitation. (c) The dimethylamino group of P9 is thought to take on a positive charge in the excited state and interact with the aromatic group in Trp141 via a cation−π interaction. (d) Fluorescence decay of the Halo–P9 conjugate (green), P9 in DBPS buffer (red), P9 in 1,4-dioxane (purple), and P9 in benzene (blue). P9 (20 μM) was incubated in DPBS buffer, benzene, 1,4-dioxane, or purified Halo protein (50 μM) for 1 h at 25 °C. (e) Electrostatic potential map of Trp141 in the ground state (left), P9 in ground state S0 (middle), and P9 in excited state S3 (right). (f) Geometric model of the cation−π interaction. (g) The left panels shows the simplified geometry of the ground state with θ = 9° and R = 3.8 Å, where the magenta “atom” represents the center of the benzene ring. The right panel shows the simplified geometry at the relaxed excited state with θ = 3° and R = 4.2 Å, exhibiting a canonical cation−π geometry.