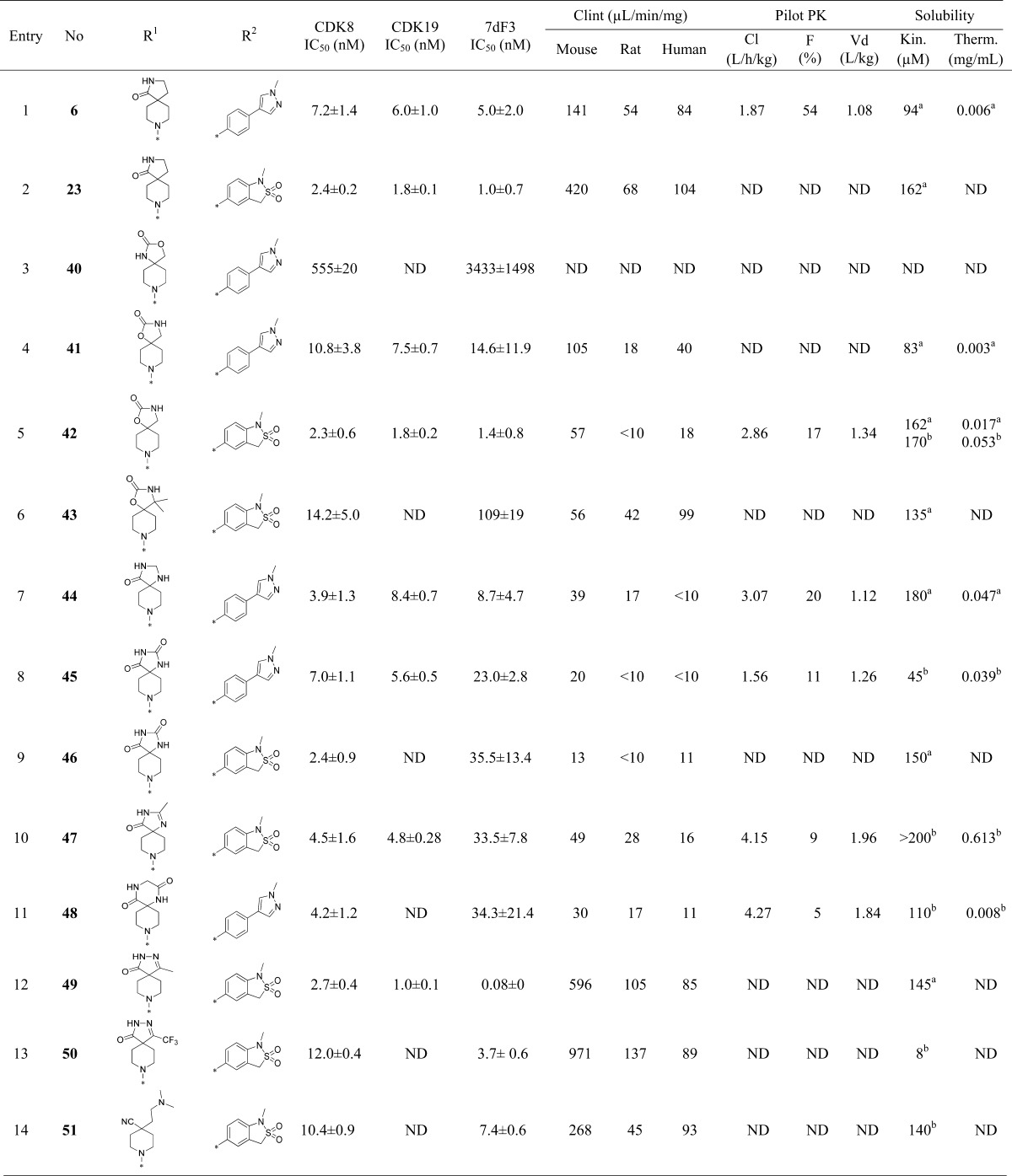
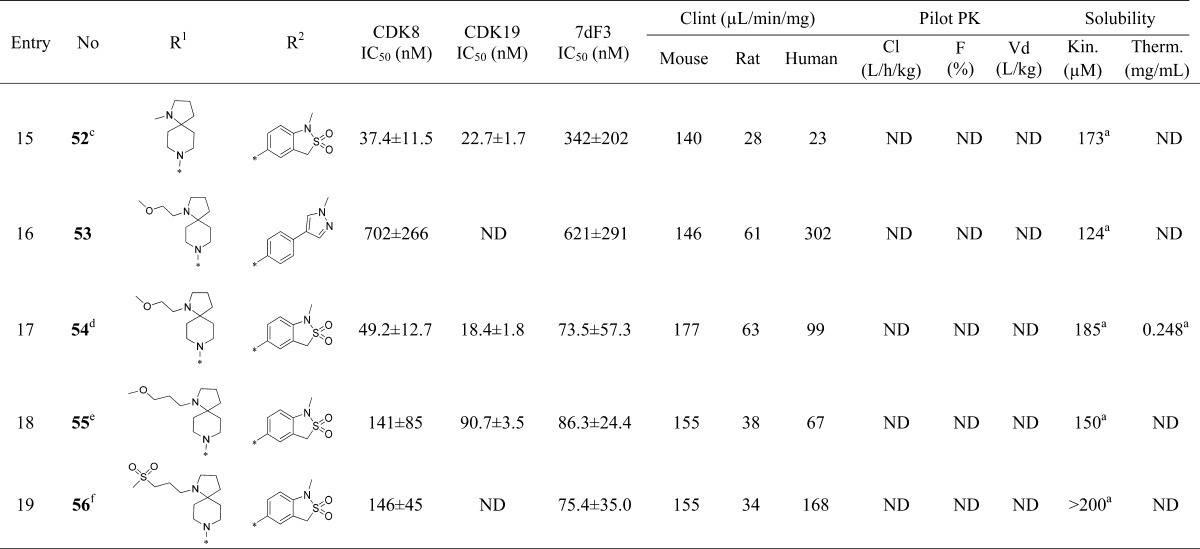
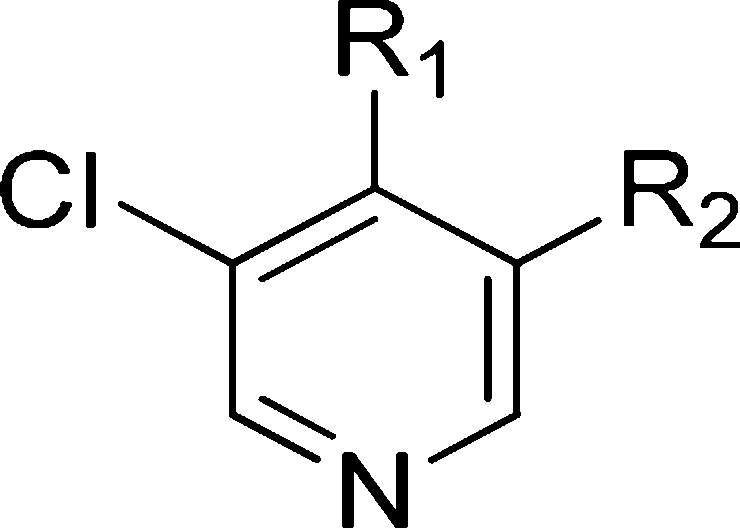
Table 3. Introduction of Polarity at the Pyridine C-4 Substituent.
Free base.
TFA salt.
The chloro substituent is at C-5 according to 5-(5-chloro-4-(1-methyl-1,8-diazaspiro[4.5]decan-8-yl)pyridin-3-yl)-1-methyl-1,3-dihydrobenzo[c]isothiazole 2,2-dioxide.
The chloro substituent is at C-5 according to 5-(5-chloro-4-(1-(2-methoxyethyl)-1,8-diazaspiro[4.5]decan-8-yl)pyridin-3-yl)-1-methyl-1,3-dihydrobenzo[c]isothiazole 2,2-dioxide.
The chloro substituent is at C-5 according to 5-(5-chloro-4-(1-(3-methoxypropyl)-1,8-diazaspiro[4.5]decan-8-yl)pyridin-3-yl)-1-methyl-1,3-dihydrobenzo[c]isothiazole 2,2-dioxide.
The chloro substituent is at C-5 according to 5-(5-chloro-4-(1-(3-(methylsulfonyl)propyl)-1,8-diazaspiro[4.5]decan-8-yl)pyridin-3-yl)-1-methyl-1,3-dihydrobenzo[c]isothiazole 2,2-dioxide.