Abstract
Many Proteobacteria possess a nitrogen-metabolic phosphotransferase system (PTSNtr) consisting of EINtr, NPr, and EIIANtr (encoded by ptsP, ptsO, and ptsN, respectively). The PTSNtr plays diverse regulatory roles, but the substrate phosphorylated by EIIANtr and its primary functions have not yet been identified. To comprehensively understand the roles of PTSNtr in Salmonella Typhimurium, we compared the whole transcriptomes of wild-type and a ΔptsN mutant. Genome-wide RNA sequencing revealed that 3.5% of the annotated genes were up- or down-regulated by three-fold or more in the absence of EIIANtr. The ΔptsN mutant significantly down-regulated the expression of genes involved in vitamin B12 synthesis, 1,2-propanediol utilization, and propionate catabolism. Moreover, the invasiveness of the ΔptsN mutant increased about 5-fold when 1,2-propanediol or propionate was added, which was attributable to the increased stability of HilD, the transcriptional regulator of Salmonella pathogenicity island-1. Interestingly, an abundance of 1,2-propanediol or propionate promoted the production of EIIANtr, suggesting the possibility of a positive feedback loop between EIIANtr and two catabolic pathways. These results demonstrate that EIIANtr is a key factor for the utilization of 1,2-propanediol and propionate as carbon and energy sources, and thereby modulates the invasiveness of Salmonella via 1,2-propanediol or propionate catabolism.
Most Proteobacteria possess the regulatory nitrogen-metabolic phosphotransferase system (PTSNtr), which operates in parallel with the phosphoenolpyruvate (PEP)-dependent carbohydrate PTS1,2,3. PTSNtr is composed of the proteins EINtr (encoded by ptsP) and NPr (encoded by ptsO) and the final phosphate acceptor EIIANtr (encoded by ptsN). In this system, three proteins form a phosphorylation chain working in the sequential order EINtr → NPr → EIIANtr4,5 (Supplementary Fig. S1). EIIANtr is known to play various regulatory roles relevant to potassium (K+) transport6,7, phosphate homeostasis8, σ factor selectivity9, fluxes through carbohydrate pathways and central metabolism10,11, stringent response12,13, and virulence14,15. For instance, EIIANtr maintains intracellular K+ homeostasis by binding to the low-affinity K+ transporter TrkA and the sensor histidine kinase KdpD in the high-affinity K+ transporting system in Escherichia coli6,7, which results in K+-mediated global gene regulation in association with both σD- and σS-dependent promoters16. Dephosphorylated EIIANtr is required for full adaptation to phosphate limitation conditions through direct interaction with the sensor kinase PhoR in a two-component system involved in inorganic phosphate homeostasis8, and it also controls the connections between C metabolism and many other cellular functions by binding to pyruvate dehydrogenase (PDH), which generates acetyl-CoA from pyruvate17. It has recently been revealed that, in Caulobacter crescentus, EIIANtr inhibits the hydrolase activity of SpoT by direct interaction13, which influences the cellular accumulation of (p)ppGpp, an alarmone controlling bacterial cell cycle progression, growth, and virulence, for its adaptation to environmental changes. In addition, EIIANtr is associated with virulence in Legionella pneumophila15 and Salmonella enterica14.
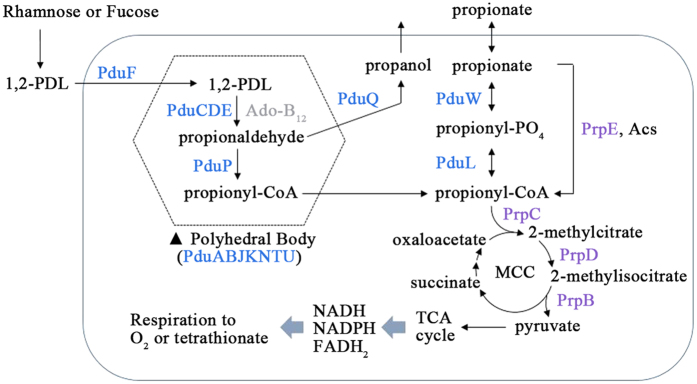
Salmonella enterica serovar Typhimurium is a bacterial pathogen that can infect a wide range of animals and causes food-borne gastroenteritis in millions of people worldwide18,19. In an animal intestinal tract, Salmonella competes with the resident bacteria occupying colonization niches, and it has evolved to harness diverse metabolic pathways to enhance its fitness during infection20. For instance, Salmonella can acquire carbon sources from 1,2-propanediol (1,2-PDL), an abundant fermentation product derived from the plant sugars L-rhamnose and L-fucose. 1,2-PDL is degraded into propionaldehyde by vitamin B12-dependent 1,2-propanediol dehydratase and further processed to 1-propanol and propionate with the coordinated action of pdu operon products21,22 (Fig. 1). In animal intestines, propionate is also provided at a high concentration as a fermentation byproduct of microflora23. Salmonella with the prp operon converts propionate to propionyl-Coenzyme A (propionyl-CoA) and further degrades into pyruvate and succinate via the 2-methylcitric acid cycle (MCC)24,25 (Fig. 1). Propionyl-CoA is therefore known as a common intermediate linking the 1,2-PDL and propionate catabolism circuits26.
Figure 1. Catabolism of 1,2-PDL and propionate in S. Typhimurium.
1,2-PDL catabolism is achieved by the 1,2-PDL utilization operon, indicated in blue. 1,2-PDL is converted into propionyl-CoA within the polyhedral microcompartment. Propionate catabolism is accomplished by the propionate operon, indicated in purple. The common intermediate propionyl-CoA provides pyruvate through the 2-methylcitrate cycle (MCC), which can be used as an energy source. Oxygen or tetrathionate is used as an electron acceptor under aerobic or anaerobic conditions, respectively.
In addition to being a metabolic intermediate for energy production, propionyl-CoA serves as a regulatory signal compromising the stability of HilD, the transcriptional regulator of Salmonella pathogenicity island-1 (SPI-1). SPI-1, a chromosomal region composed of 39 genes, encodes a type 3 secretion system (T3SS), whereby its cognate effector proteins are translocated into host cells to promote bacterial invasion. An increase in propionyl-CoA production compromises the stability and activity of HilD in a post-translational manner, thus attenuating Salmonella invasion into intestinal epithelial cells27. Although SPI-1 is prominently responsible for Salmonella invasion into host cells, SPI-4 is also required for bacterial adhesion to polarized epithelial cells as well as for invasion28. The SPI-4 locus contains six genes, siiABCDEF, forming an operon. SiiE, which is secreted via an SPI-4-encoded type 1 secretion system (SPI-4 T1SS), facilitates Salmonella adhesion to polarized host cells. Not surprisingly, the expression of SPI-4 is coordinated with SPI-1 regulation. SPI-1 uses its cognate regulators (HilD, HilC, HilA, and InvF) for its systematic regulation. HilD and HilC induce the expression of HilA in combination or independently. HilA, in turn, directly activates the expression of invF and the genes encoding T3SS and also indirectly induces the transcription of SPI-4. HilA antagonizes the H-NS-mediated transcriptional silencing of SPI-429.
To better understand the roles of EIIANtr, we used RNA-seq to compare the whole transcriptomes of the wild-type and a mutant strain lacking ptsN. Genes involved in vitamin B12 synthesis, 1,2-PDL utilization, and propionate catabolism were significantly down-regulated in the ΔptsN mutant strain, whereas SPI-1 and SPI-4 were up-regulated. In accordance with the transcriptional differences, the ΔptsN mutant strain was more competent in invasion into host cells than the wild-type bacteria when they were pre-cultured with 1,2-PDL or propionate. We found that EIIANtr can control Salmonella invasion by balancing propionyl-CoA in response to environmental carbon metabolites.
Results
Comprehensive understanding of the roles of EIIANtr in Salmonella using transcriptome analysis
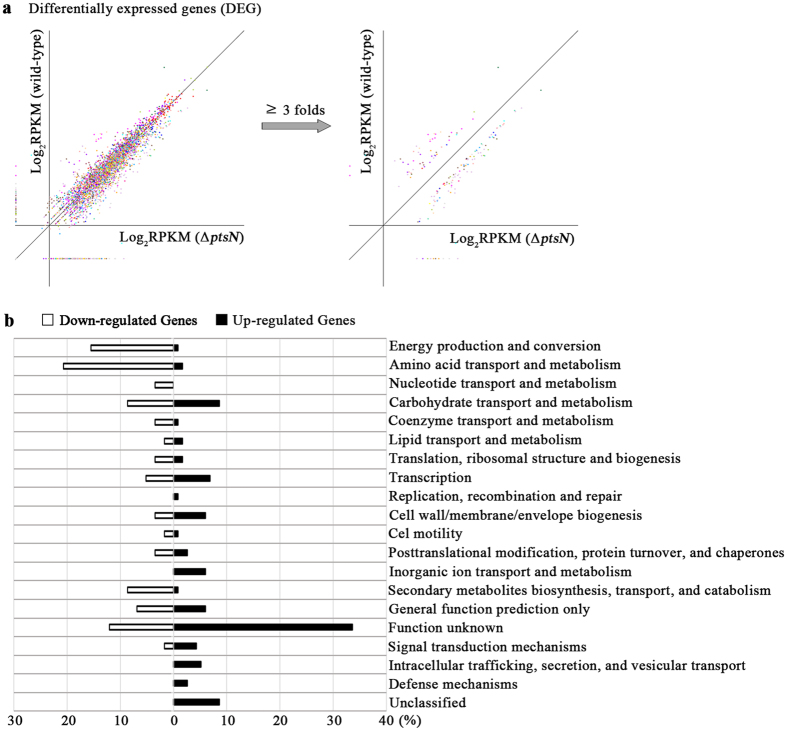
EIIANtr plays multifaceted regulatory roles in diverse bacterial species, including Escherichia coli6,7, Pseudomonas putida30 and Salmonella Typhimurium14. In this study, transcriptome profiling of a Salmonella ΔptsN mutant strain was conducted to understand the comprehensive roles of EIIANtr in Salmonella metabolism and virulence. The ΔptsN mutant strain did not show growth defects in LB broth at 37 °C compared to its parent strain, S. Typhimurium SL1344 (Supplementary Fig. S2a and S2b). Total RNA was extracted at a mid-exponential phase of growth (OD600 = 1) and analyzed using RNA-seq. Mapping analysis of RNA-seq data was summarized in Supplementary Table S1. A statistical comparison between the two strains (p value < 0.05) revealed that 2.40% (116/4837) and 1.12% (54/4837) of the total genes were up- and down-regulated, respectively, by 3-fold or more in the ΔptsN mutant strain compared to the wild-type (Fig. 2a). The differentially expressed genes (DEGs) were categorized based on their predicted functions by cluster of orthologous group designations31. Genes with functions in energy production and conversion, amino acid transport and metabolism, coenzyme transport and metabolism, and secondary metabolites biosynthesis/transport/catabolism were down-regulated overall in the absence of EIIANtr, and genes involved in cell wall/membrane/envelope biogenesis, inorganic ion transport and metabolism, signal transduction, intracellular trafficking/secretion/vesicular transport, and defense mechanisms were generally up-regulated (Fig. 2b). The likelihood of multiphasic cellular regulation by EIIANtr was predicted from the previously identified roles of EIIANtr in K+ homeostasis6,7, phosphate starvation responses8, ppGpp synthesis/hydrolysis12, amino acid synthesis/metabolism32,33, and carbon metabolism17. Taken together, these results suggest that EIIANtr affects diverse metabolisms associated with Salmonella fitness.
Figure 2. Classification of differentially expressed genes (DEGs) based on predicted functions.
(a) DEG analysis from RNA-seq data between wild-type Salmonella and the ΔptsN mutant strain. The y-axis shows the log-scaled RPKM values of wild-type, and the x-axis shows the log-scaled RPKM values of the ΔptsN mutant strain. Total gene expressions in the two strains (left) were filtered to sort significantly down- or up-regulated genes (right) with the criteria of p-value ≤ 0.05 and fold-change ≥3. (b) Genes up-regulated or down-regulated by 3-fold or more in the ΔptsN mutant strain are grouped into functional categories. Genes are annotated based on the cluster of orthologous groups database.
EIIANtr activated expression of genes involved in 1,2-PDL utilization and propionate catabolism
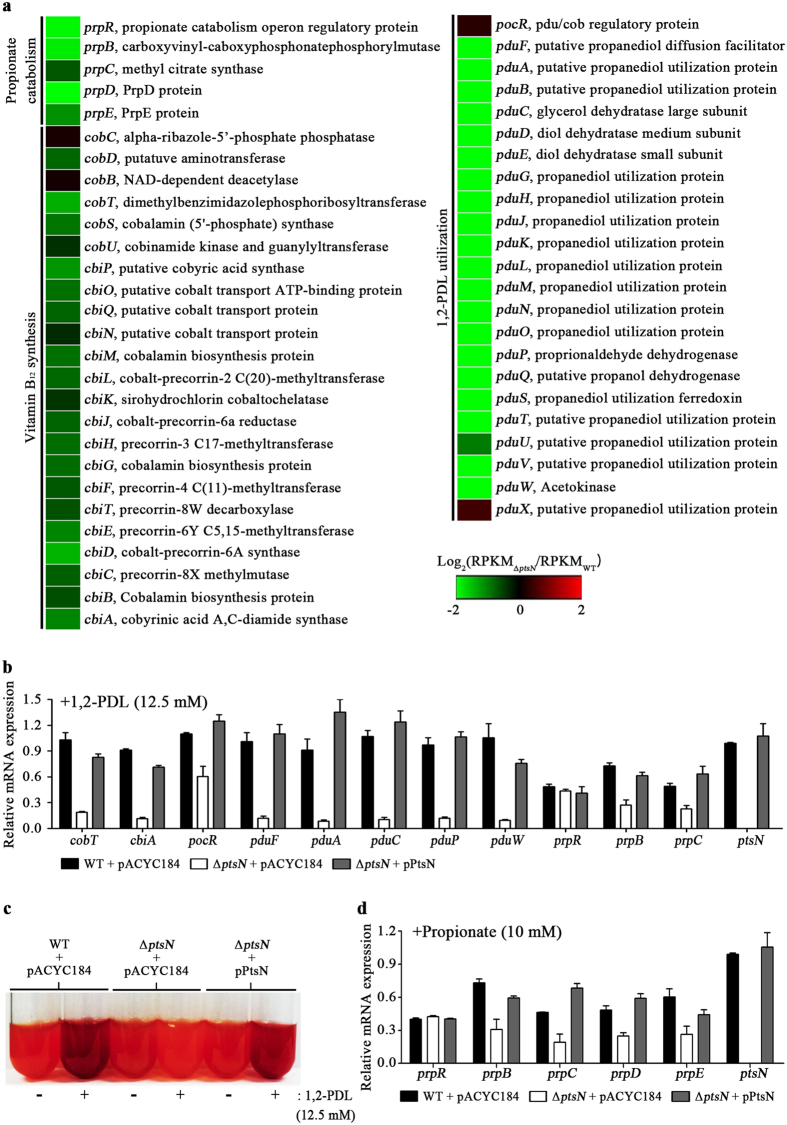
An effort to make sense of the primary roles of EIIANtr in Salmonella led to a search for genes coordinately controlled as an operon by EIIANtr. Genes constituting the 1,2-PDL utilization (pdu) operon mostly decreased in the ΔptsN mutant strain, indicating a positive role of EIIANtr in utilizing 1,2-PDL as a carbon source (Fig. 3a; Supplementary Table S2). The pdu operon consists of 21 genes, with pocR and pduF encoding its cognate activator and membrane transporter, respectively, in the opposite direction on the chromosome and encodes proteins for the catabolism of 1,2-PDL. Salmonella degrades 1,2-PDL into propionaldehyde with the aid of the cofactor adenosyl cobalamin (Ado-B12) and further processes it into propanol and propionate through propionyl-CoA to acquire energy in nutrient-restricted conditions22. In accordance with a decrease in pdu expression, the cob-cbi operon encoding enzymes for the biosynthesis of Ado-B12 was inclined toward down-regulation by the absence of EIIANtr (Fig. 3a; Supplementary Table S2). Propionyl-CoA is a common intermediate linking the 1,2-PDL and propionate catabolic pathways (Fig. 1). Propionyl-CoA, produced in the middle of the 1,2-PDL degradation pathway or synthesized from propionate by multiple propionyl-CoA-synthesizing systems, is integrated into the MCC and further catabolized to pyruvate and succinate through the coordinated action of prp operon-encoded enzymes24,25. Thus, the 1,2-PDL and propionate catabolic pathways enable Salmonella to produce energy and carbon sources and outcompete commensal microbiota in a nutrient-restricted host intestine20. The transcriptome profiling revealed that four genes of the prp operon and their regulator gene prpR, which are transcribed divergently on the chromosome, were down-regulated in step with the pdu and cob-cbi operons in the ΔptsN mutant strain (Fig. 3a; Supplementary Table S2), indicating that EIIANtr coordinately activates transcription of multiple genes to exploit 1,2-PDL and propionate, which are disfavored carbon sources for the bystander microbiota20. The levels of mRNAs relevant to vitamin B12 synthesis (cobT and cbiA), 1,2-PDL utilization (pocR, pduF, pduA, pduC, pduP, and pduW), and propionate catabolism (prpB and prpC) were measured using qRT-PCR to verify the RNA-seq results. The lack of EIIANtr decreased the expression of genes involved in vitamin B12 synthesis and 1,2-PDL and propionate catabolic pathways in accordance with the transcriptome analysis (data not shown). As expected, when Salmonella strains were supplemented with 1,2-PDL and Ado-B12, genes of the cob-cbi, pdu, and prp operons were much less transcribed in the ΔptsN mutant strain than in the wild-type, and those decreases were complemented with trans-encoded EIIANtr by the pPtsN plasmid (Fig. 3b).
Figure 3. The effects of ptsN on the expression of genes involved in propionate catabolism, vitamin B12 synthesis, and 1,2-PDL utilization.
(a) Heat-map analysis of the expression ratios of the prp, cob-cbi, and pdu genes between the ΔptsN mutant and wild-type strains (log2[ΔptsN mutant/wild-type]). (b) Evaluation of mRNA levels of the cob-cbi, pdu, and prp genes in strains of wild-type, ΔptsN, and ΔptsN containing pPtsN. Every mRNA level was normalized using that of gyrB in each strain and its relative expression was estimated comparing with the mRNA level of ptsN in wild-type containing pACYC184, which was set at 1.0. The pPtsN plasmid has ptsN and its putative promoter sequences on the backbone of pACYC184. Ado-B12 (20 nM) and 1,2-PDL (12.5 mM) were added to the culture to stimulate the prp operon under aerobic conditions. (c) Medium pH was assayed to compare the activity of 1,2-PDL catabolism in wild-type SL1344 containing pACYC184 and the ΔptsN mutant strain harboring pPtsN or pACYC184. Neutral-red (0.0033%) was added to the culture as a pH indicator and changed its color to dark red in proportion to the level of propionate, a byproduct of the 1,2-PDL pathway. All cultures were supplemented with Ado-B12 (20 nM), and 1,2-PDL (12.5 mM) was added as a substrate for the 1,2-PDL pathway. The result shown is representative of three independent tests. (d) The mRNA levels of prp genes were measured in a similar manner as conducted in (b) with normalization to the mRNA level of ptsN in the wild-type containing pACYC184, but propionate (10 mM) was added as an alternative carbon source instead of 1,2-PDL and Ado-B12.
EIIANtr negatively controls expression of SPI-1 and SPI-4, which are important for Salmonella invasion
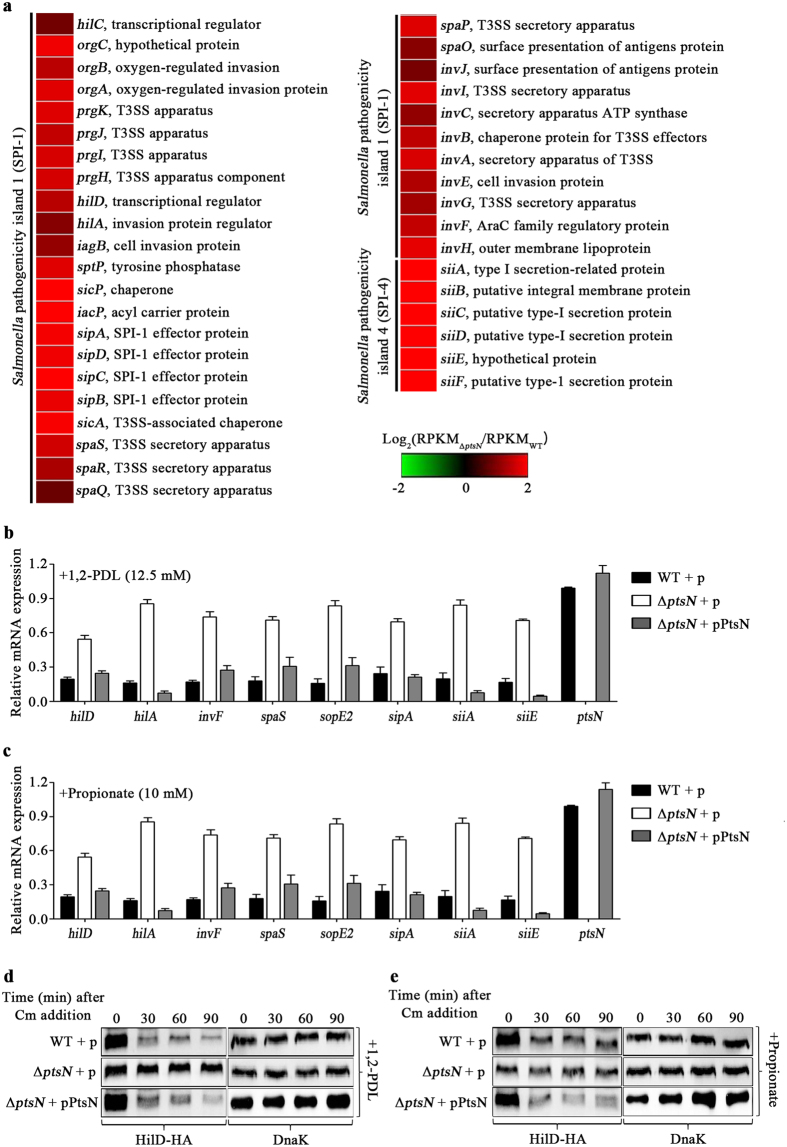
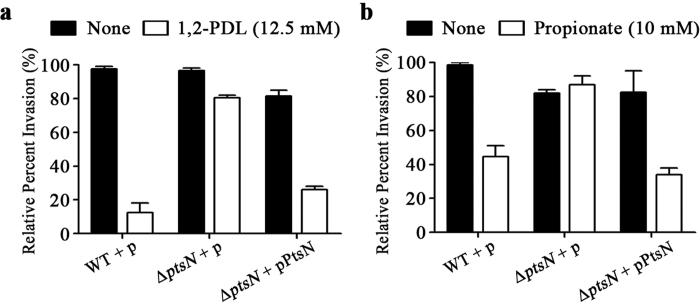
Recently, propionyl-CoA, the intermediate in the 1,2-PDL and propionate catabolic pathways, was found to function as a regulatory signal. Intracellular propionyl-CoA synthesized from the propionate abundant in the host intestine attenuated the stability and activity of HilD, the master regulator of SPI-1, which led to reduced Salmonella invasion into epithelial cells27. In agreement with that observation, altered expression of the pdu and prp operons, which depends on the presence of EIIANtr, also influenced the expression of SPI-1 and SPI-4 (Fig. 4a; Supplementary Table S2). The ΔptsN mutant strain more weakly degraded 1,2-PDL into propionyl-CoA and propionate than did wild-type Salmonella, turning the neutral-red, an acidic pH indicator, light red instead of dark red like the wild-type strain (Fig. 3c). A reduction in propionyl-CoA in the ΔptsN mutant strain presumably activates SPI-1 expression through the accumulation of HilD, which in turn stimulates SPI-4 expression via HilA. Differential expression of the SPI-1 and SPI-4 genes depending on the presence of EIIANtr was also validated using qRT-PCR (Fig. 4b and c). When 1,2-PDL or propionate was added to the culture for conversion into propionyl-CoA, the ΔptsN mutant strain increased SPI-1 and SPI-4 expression, and that altered expression was reversed by the introduction of pPtsN, producing EIIANtr in trans. Thus, the capacity to use 1,2-PDL and propionate as carbon sources influences Salmonella invasiveness into epithelial cells (Fig. 5). The presence of 1,2-PDL and propionate attenuated the invasiveness of Salmonella wild-type strains, probably through an increase in propionyl-CoA via the 1,2-PDL and propionate catabolic pathways, whereas the differential invasion ability in response to 1,2-PDL and propionate was abolished in the absence of EIIANtr. Complementing the ΔptsN mutant strain with pPtsN producing EIIANtr in trans restored the ability to control invasiveness depending on the presence of 1,2-PDL or propionate. Consequently, this result suggests that EIIANtr influences the ability of Salmonella to invade into host cells by manipulating propionyl-CoA production when 1,2-PDL or propionate is abundant.
Figure 4. The effects of ptsN on the expression of genes involved in bacterial invasiveness.
(a) Heat-maps generated to describe the expression patterns of SPI-1 and SPI-4 genes in the ΔptsN mutant strain. Gene expression ratios between the ΔptsN mutant and wild-type SL1344 are shown using a colorimetric gradient: down-regulation in green and up-regulation in red. (b,c) The relative mRNA levels of the genes involved in SPI-1 and SPI-4, as measured by qRT-PCR. The tested genes are the SPI-1 regulator genes (hilD, hilA, and invF), SPI-1 genes (spaS, sopE2, and sipA), and SPI-4 genes (siiA and siiE). As alternative carbon sources, Ado-B12 (20 nM), 1,2-PDL (12.5 mM), and propionate (10 mM) were added to the cultures of (b and c), respectively. The mRNA level of each gene was normalized using that of gyrB in the wild-type strain containing pACYC184 and the ΔptsN mutant strains with either pACYC184 or pPtsN and its relative expression was calculated using the mRNA level of ptsN in the wild-type containing pACYC184, which was set at 1.0. All experiments were performed in triplicate, and the average values are depicted in the graphs. (d and e) The stability of the HilD-HA protein, compared under conditions identical to those used in (b) and (c). Three strains of wild-type with pACYC184 and ΔptsN mutant strains with either pACYC184 or pPtsN were cultivated in the presence of 1,2-PDL (d) or propionate (e). De novo protein synthesis was quenched by the addition of chloramphenicol (0.2 mg/ml) at 4 h post inoculation. Total proteins were harvested every 15 min, and equivalent amounts were subjected to SDS-PAGE analysis. The stability of HilD-HA was assessed using anti-HA antibody. The levels of DnaK as controls were comparable between lanes. Full-length blots were presented in Supplementary Figure S5.
Figure 5. Invasion of epithelial cells by Salmonella in the presence of 1,2-PDL or propionate.
(a) Three S. Typhimurium strains, wild-type, the ΔptsN mutant, and the ΔptsN mutant containing pPtsN, were pre-cultured with Ado-B12 (20 nM) and 1,2-PDL (12.5 mM) or not prior to infection and added to monolayers of Caco-2 cells at an MOI of 10. At 1.5 h post infection, cells were lysed, and intracellular bacteria were enumerated by plating. Values represent the relative amount of internalized bacteria and are normalized to the level of internalization of the wild-type containing the empty vector, which was set at 100%. (b) A similar invasion test was conducted, but propionate (10 mM) was provided as an alternative carbon source pre-cultivation. All invasion tests were carried out independently at least three times, and the averaged values are shown with standard deviations.
Dissecting EIIANtr roles in transcriptional regulation of pdu and prp operons and SPI-1 and -4
The 1,2-PDL and propionate catabolic pathways are tightly linked by their use of propionyl-CoA as a common intermediate. The two pathways together accomplish the conversion between propionate and propionyl-CoA (Fig. 1). In this context, the influence of EIIANtr on prp expression might be indirect, mediated by the activity of the 1,2-PDL pathway discharging propionyl-CoA and propionate into the propionate pathway. The addition of 1,2-PDL led to increases in prp and pdu expression, and the lack of EIIANtr diminished expression of both prp and pdu (Fig. 3b and Supplementary Fig. S3b). On the other hand, the addition of propionate activated prp genes but not pdu genes (Fig. 3d and Supplementary Fig. S3). Furthermore, the prp operon was activated in response to propionate even when the 1,2-PDL pathway was blocked (Supplementary Fig. S3b), indicating the possibility of diverting the propionate pathway from 1,2-PDL depending on available carbon sources. The positive role of EIIANtr in prp operon expression was also achieved in propionate-supplemented conditions, where the 1,2-PDL pathway was suspended (Fig. 3d and Supplementary Fig. S3b). Thus, EIIANtr likely controls the 1,2-PDL and propionate catabolic pathways in a pleiotropic manner in response to carbon sources: coordinated activation of two pathways in the presence of 1,2-PDL and localized activation of the propionate pathway in the presence of propionate only.
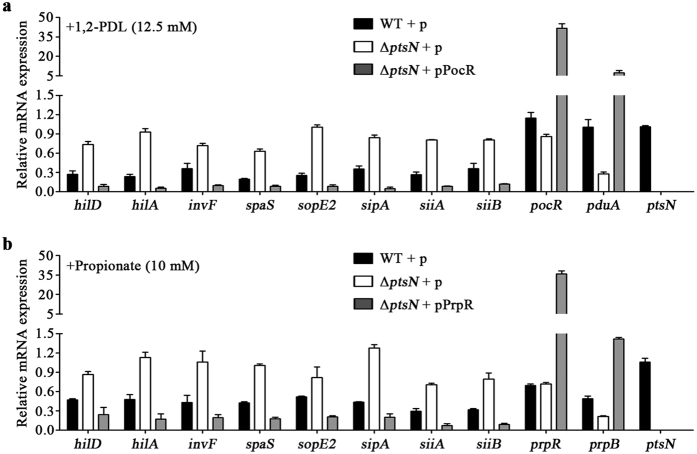
Propionyl-CoA is known to reduce Salmonella SPI-1 expression by destabilizing HilD and lowering its activity post-translationally27. SPI-1 expression is tightly controlled by a multitude of regulators, including HilD, HilC, RtsA, HilA, and InvF, though HilD holds predominant control of the regulatory circuit34,35. SPI-4, which is activated by HilA, is coupled with SPI-1 in the context of transcriptional regulation and cellular function and stimulates Salmonella invasion into host cells in concert with SPI-129. Therefore, the increased expression of SPI-1 and SPI-4 in the ΔptsN mutant strain was likely attributable to the HilD that accumulated at low propionyl-CoA levels. To examine this possibility, the stability of the HilD protein was compared between the wild-type and ΔptsN mutant strains in the presence of 1,2-PDL or propionate as a precursor for propionyl-CoA. The ΔptsN mutant strain maintained HilD continually for at least 90 min after chloramphenicol addition, whereas wild-type Salmonella degraded HilD gradually (Fig. 4d and e). Furthermore, the negative role of EIIANtr in SPI-1 and SPI-4 expression was reversible by the overexpression of PocR and PrpR, the cognate activators for the pdu and prp operons, respectively (Fig. 6a and b). These results suggest that EIIANtr affects the expression of SPI-1 and SPI-4 indirectly by modulating HilD post-translationally via the 1,2-PDL and propionate catabolic pathways.
Figure 6. The effects of PocR or PrpR overexpression on SPI-1 and SPI-4 in the ΔptsN mutant strain in the presence of 1,2-PDL or propionate.
(a) The relative mRNA expression of genes involved in SPI-1 and SPI-4 was determined by qRT-PCR in the wild-type containing pUHE21-2lacIq and in the ΔptsN mutant strains harboring either pUHE21-2lacIq or pPocR. pocR, which encodes the transcriptional activator for the pdu operon, was induced by the addition of 1 mM IPTG from pPocR. Strains were grown in LB medium containing 1,2-PDL (12.5 mM) as an additional carbon source. (b) A similar qRT-PCR was performed, but pPrpR producing PrpR, the transcriptional activator of the prp operon, was used instead, and propionate (10 mM) was added accordingly. Every mRNA level was normalized using that of gyrB and its relative expression to ptsN was averaged from three assays at least.
Translational levels of EIIANtr are up-regulated in response to 1,2-PDL and propionate
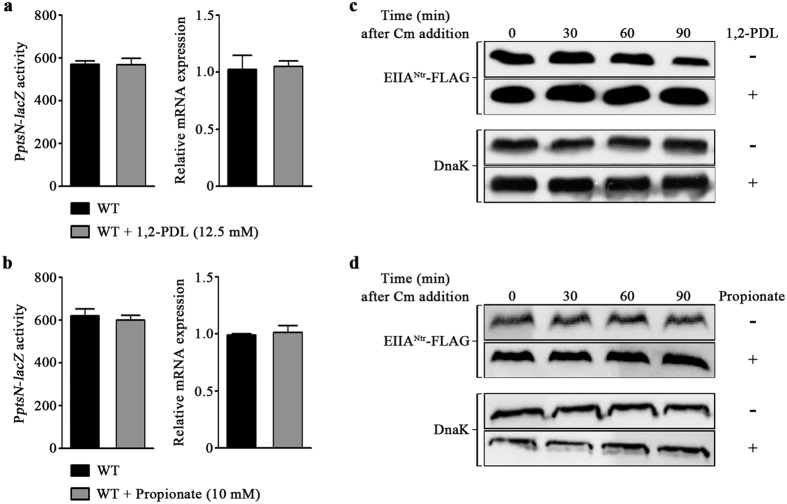
ptsN is a component of the rpoN operon, which is co-transcribed with rpoN from a single promoter upstream of rpoN. Because rpoN-encoded sigma 54 (σ54) controls the transcription of a plethora of genes involved in nitrogen assimilation and stress responses, PTSNtr has been considered as a regulatory system associated with nitrogen metabolism2,3. However, it remains unclear which stimuli trigger the activation of PTSNtr. Intracellular balance between nitrogen and carbon was found to modulate the phosphorylation status of EIIANtr, the output regulator of PTSNtr36. In addition, the availability of amino sugar was recently revealed to control the degradation rate of EIIANtr37. The finding that the positive role of EIIANtr in pdu or prp expression is remarkable only in the presence of 1,2-PDL or propionate prompted analysis of whether the expression or activity of EIIANtr could be enhanced in response to 1,2-PDL or propionate. The expression level of ptsN was measured in two different ways: a β-gal assay using lacZ fusion to the promoter of ptsN and ptsN mRNA quantification using qRT-PCR. The transcription of ptsN was unaffected by 1,2-PDL or propionate (Fig. 7a and b). However, the protein level of EIIANtr was significantly higher in the presence of 1,2-PDL (Fig. 7c) or propionate (Fig. 7d). To rule out the possibility that the different EIIANtr levels were caused by accelerated degradation of EIIANtr in the absence of 1,2-PDL or propionate, the stability of EIIANtr was compared after quenching protein synthesis using chloramphenicol. Degradation rates of EIIANtr were not influenced by the presence of 1,2-PDL or propionate, showing comparable protein levels for 90 min (Fig. 7c and d). Thus, ptsN mRNA might be translated into EIIANtr with different efficiency depending on the abundance of 1,2-PDL or propionate.
Figure 7. EIIANtr production increased in response to 1,2-PDL and propionate.
(a and b) The transcriptional expression of ptsN was measured with or without 1,2-PDL (a) and propionate (b). β-Galactosidase activity from PptsN::lacZ was measured in Miller units (left), and the relative ptsN mRNA expression was determined using qRT-PCR (right). Each bar represents the average value of three independent experiments. (c and d) The stability of EIIANtr-FLAG was evaluated by western blot analysis with or without 1,2-PDL (12.5 mM) (c) and propionate (10 mM) (d). At 5 h post inoculation, mRNA translation was halted by adding chloramphenicol (0.2 mg/ml), and that time was set as 0 min. Total proteins were harvested every 30 min, and equivalent amounts were subjected to SDS-PAGE analysis. The stability of EIIANtr-FLAG was assessed using an anti-FLAG antibody. The levels of DnaK were used as controls. Full-length blots were presented in Supplementary Figure S6.
Discussion
In this study, we found that ptsN-encoded EIIANtr, a component of the nitrogen-metabolic PTS, is a key factor controlling vitamin B12 synthesis, 1,2-PDL utilization, propionate catabolism, and invasion in S. Typhimurium. Transcriptome analysis using RNA-seq revealed that a lack of EIIANtr decreased the expression of genes required for vitamin B12 synthesis, 1,2-PDL utilization, and propionate catabolism, and we validated that observation by qRT-PCR using total RNA extracted from cultures grown in the presence of 1,2-PDL or propionate. In accordance with a previous report about the negative regulation of SPI-1 by propionyl-CoA27, we found that the down-regulation of the 1,2-PDL and propionate pathways in the ΔptsN mutant strain alleviated the rapid degradation of HilD, a dominant SPI-1 activator, and consequently led to an increase in invasion ability of Salmonella.
EIIANtr has been reported to exert its regulatory activity via direct protein-protein interactions6,7,8,13,14. In an effort to understand how EIIANtr orchestrates 1,2-PDL and propionate catabolism, we examined the possibility of direct protein-protein interaction between EIIANtr and various regulatory factors associated with both catabolism using bacterial two-hybrid system38. Four different proteins including regulators of 1,2-PDL and propionate operons (PocR and PrpR, respectively), cAMP receptor protein (CRP), and adenylyl cyclase (AC) were tested but none of them showed interaction with EIIANtr (unpublished data). CRP and AC are involved in the regulation of cobalamin regulon as well as 1,2-PDL and propionate operons39,40. However, it cannot be ruled out that other regulatory factors might coordinate the interaction between EIIANtr and 1,2-PDL or propionate catabolism directly or indirectly.
S. Typhimurium causes non-typhoid gastroenteritis in humans and typhoid-like disease in mice41,42. During infection, Salmonella invades intestinal epithelial cells and replicates inside macrophages43,44. The ability to acquire nutrients during the infection process is crucial for enteric pathogens to survive and proliferate in the host because they have to compete with commensal microbiota for limited nutrients20. With regard to bacterial competition for restricted carbon sources, S. Typhimurium outcompetes commensal bacteria through two distinctive but closely linked pathways: the 1,2-PDL utilization pathway and the propionate catabolic pathway20. Plant sugars such as L-rhamnose and L-fucose, which are abundant in digested food molecules, are degraded into 1,2-PDL by gut microbiota. L-fucose constitutes mucosal glycoconjugates as a terminal sugar of the oligosaccharide chains linked to the mucin protein backbone and is easily accessible to enteric bacteria in the intestinal lumen22,45. Propionate is also provided at high concentrations through several fermentation pathways in human gut bacteria46. However, only enteropathogenic Enterobacteriaceae, including S. Typhimurium, can use 1,2-PDL and propionate as carbon sources to generate energy47. S. Typhimurium harnesses the 1,2-PDL and propionate catabolic pathways to convert those less-favored carbon sources into pyruvate and eventually ATP.
When Salmonella resides in an oxygen-abundant milieu, propionyl-CoA is integrated into the MCC, coupled with the citric acid cycle, and processed to release energy via an electron-transport chain that uses oxygen as the final electron acceptor. Meanwhile, in anaerobic conditions like the distal gut, Salmonella exploits the cob, pdu, and prp operons in concert with the ttr operon for anaerobic respiration using tetrathionate as the final electron acceptor instead48. The cob-cbi operon encodes factors required for vitamin B12 synthesis only under anaerobic conditions49. Ado-B12 is then used as a cofactor in the 1,2-PDL pathway to catabolize 1,2-PDL to propionyl-CoA in combination with propanol and propionate. Propionyl-CoA is further processed via the MCC and the citric acid cycle, as in aerobic conditions, whereas metabolic byproducts such as NADH and its equivalents are oxidized sequentially with tetrathionate as the electron acceptor. Tetrathionate, usually present in humid soils, has recently been found to be produced during the inflammation response to enteropathogenic infection. Hydrogen sulfide (H2S), produced by colonic bacteria, is detoxified to thiosulfate (S2O32−) and further oxidized to tetrathionate (S4O62−) by nitric oxide radicals and reactive oxygen species generated in the intestinal lumen50. In contrast to coliforms, which are inactivated by tetrathionate, S. Typhimurium possesses the ttr operon composed of ttrABCRS, which enables it to exploit tetrathionate as an electron acceptor in the respiratory chain. This process gives S. Typhimurium a competitive edge over gut microbiota and allows it to outgrow commensal bacteria and ultimately to achieve transmission to new recipients51,52.
Considering the opposite roles of EIIANtr vis-à-vis the cob-cbi, pdu, and prp operons and SPI-1 and SPI-4 regulation, S. Typhimurium in an intestinal lumen enriched with 1,2-PDL and propionate seems to inevitably produce propionyl-CoA and thus lower the expression of SPI-1 and SPI-4, which are critical for Salmonella invasion into epithelial cells. Fine-tuning of Salmonella virulence by EIIANtr has been reported in the regulation of SPI-2, a Salmonella pathogenicity island essential for the proliferation of Salmonella in macrophages53. ssrA/ssrB, which encode the two-component regulatory system of SPI-2, are expressed ectopically within phagosomes in response to intracellular cues. EIIANtr directly interacts with SsrB, inhibiting SsrB from over activating the transcription of SPI-2 genes14. The differential roles of EIIANtr, which provides S. Typhimurium with a growth benefit over the competing microbiota but dampens invasion activity in the intestinal lumen abundant with 1,2-PDL and propionate, could be a sophisticated strategy for bacterial fitness inside the host. S. Typhimurium is a gastrointestinal pathovar that causes general inflammation in the human intestine but rarely disseminates into deeper tissues to cause systemic infection. However, invasive typhoidal Salmonella serovars such as S. typhi are prone to disrupt the intestinal epithelial barrier and spread over the whole body. Interestingly, the invasive Salmonella serovars evolutionally adapted for extraintestinal life are defective in anaerobic metabolic networks54. Bacterial functions attributable to the cob, pdu, prp, and ttr operons have been sequentially compromised during evolution away from an intestinal life. S. Typhimurium, adapted for intestinal life, might use EIIANtr as a regulatory switch to balance between bacterial intestinal colonization and internalization into host cells in response to the environmental nutrient repertoire.
Thus, we conclude that EIIANtr is a key player that not only controls Salmonella fitness in response to the availability of 1,2-PDL or propionate but also influences its internalization into host cells by modulating the production of a metabolic intermediate of propionyl-CoA during host infection.
Methods
Bacterial strains, media, and culture conditions
All bacterial strains were derived from S. Typhimurium SL1344 as the parent and are listed in Supplementary Table S3. Luria-Bertani (LB) medium containing 1% Bacto-tryptone, 0.5% yeast extract, and 1% NaCl (pH 7.5) was used as the complex culture medium for the routine growth of bacteria. All S. Typhimurium strains were grown aerobically at 37 °C with antibiotic supplementation at the following concentrations: ampicillin, 50 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 25 μg/ml. As an alternative carbon source, 1,2-PDL (12.5 mM) or propionate (10 mM) was added when bacterial cultures reached an optical density of 0.5–0.6 at 600 nm (OD600). In the case of 1,2-PDL utilization, Ado-B12 (20 nM) was also added as a cofactor to the LB medium.
Construction of strains and plasmids
The ptsN deletion mutant strain was constructed using the lambda red recombination method for in-frame gene deletion55. For the construction of the ΔptsN mutant strain, SR7001, the KmR cassette from pKD13 was amplified using the primers ptsN-del-F and ptsN-del-R. The resulting PCR products were integrated into the ptsN region in the SL1344 wild-type strain containing the plasmid pKD46, followed by selection for ΔptsN::kan transformants. The KmR cassette was removed using the plasmid pCP2055. For the deletion of the pdu operon, the CmR cassette from pKD3 was amplified using the primers pduA~X-del-F and pduA~X-del-R. After that, the lambda red recombination method was followed, as mentioned above. The tagging of EIIANtr and HilD with the FLAG and HA peptides, respectively, at the C-terminus was also performed using the same recombination system with each primer set: ptsN-FLAG-F/ptsN-FLAG-R and HilD-HA-F/HilD-HA-R. To construct pPtsN plasmid expressing ptsN gene, the ptsN gene was amplified by PCR using the primers ptsN-comple-F and ptsN-comple-R containing the restriction sites HindIII and SphI, respectively. The PCR product harboring the ptsN structural gene, its own promoter and RBS was introduced between the HindIII and SphI restriction sites of the pACYC184 vector. A new promoter was found at 67 base upstream of the ATG codon of the ptsN gene in addition to the one upstream of the rpoN gene in S. Typhimurium SL1344 (un-published data) anfd used for ptsN expression in pPtsN construction. To construct pPocR expressing PocR under the lac promoter, the pocR gene was amplified by PCR using the pocR-over-R and pocR-over-R primers, and the purified PCR product was inserted between the BamHI and HindIII sites of the pUHE21-2lacIq vector56. Similarly, the prpR gene was amplified using the prpR-over-F and prpR-over-R primers, and then the PCR product was inserted into the pUHE21-2lacIq vector via the BamHI and PstI sites to make pPrpR, which overexpresses PrpR. To construct the strain carrying a lacZ reporter gene, KmR cassette from pKD13 was amplified using primer sets and the lambda red recombination method. Then the lacZ gene was introduced using the plasmid pCE7057. For lacZ fusion in mutant strains, the integrated lacZ gene was transferred using bacteriophage P22 transduction58. The sequences of primers used in constructing the Salmonella strains and plasmids are listed in Supplementary Table S4.
RNA isolation and sequencing
Salmonella strains were grown in LB medium under aerobic conditions at 37 °C to the log phase with an OD600 of 1.2–1.4 (Supplementary Fig. S2). RNAprotect bacterial reagent (Qiagen, Hilden, Germany) was applied at an appropriate volume for each collected sample before total RNA extraction. Total RNA was isolated using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions, and residual DNA was removed using Ambion Turbo DNA-freeTM (ThermoFisher Scientific, Braunschweig, Germany). The quantity and quality of total RNA were examined using Agilent 2100 Bioanalyzers (Agilent Technologies, CA, USA), and the RNA integrity number (RIN) was determined. Only RNAs with a RIN > 9 were used in further experiments. The extracted total RNA was stored at −80 °C until use. All RNA-seq and alignment process were performed in Chunlab, Inc. (Seoul, Korea). Five micrograms of total RNA from each sample was used as starting material. If needed, the RNA was concentrated. The RNA was then subjected to subtractive hybridization/bead capture rRNA-removal using the Ribo-Zero kit (Epicentre Biotechnologies, WI, USA). The mRNA was fragmented ultrasonically and then converted into an RNA-seq library using the mRNA-seq library construction kit v.2 (Illumina, CA, USA) according to the manufacturer’s instructions. RNA 2 × 100 bp paired-end sequencing was performed using the Illumina GAII (Illumina) according to the manufacturer’s protocol (Supplementary Fig. S4). The genome sequence data of S. Typhimurium SL1344 for the reference genome was retrieved from the NCBI database. Quality-filtered reads were aligned with the reference genome using Bowtie259. RNA-seq was carried out once for both wild-type and a ΔptsN mutant strain. Gene expression was quantified as reads per kilobase per million mapped reads (RPKM)60. Differentially expressed genes (DEGs) with a fold change of 3 or more (p-value < 0.05) were filtered and visualized by the CLRNAseq program (Chunlab).
Heat Map Generation
A heat map was drawn to analyze the global response pattern. The log2RPKM values of relative gene expression of the Salmonella enterica serovar Typhimurium SL1344 wild-type and ΔptsN mutant strains were calculated. The heat map and hierarchical clusters were then generated using Gitools v2.2.2.
Quantitative real-time RT-PCR
The total RNA samples were treated with RNase-free DNase (ThermoFisher Scientific), and cDNA was synthesized using RNA to cDNA EcoDryTM Premix (random hexamers) (Clontech). Quantification of cDNA was carried out using 2 × iQ SYBR Green Supermix (Bio-Rad, CA, USA), and real-time amplification of the PCR products was performed using the iCycler iQ real-time detection system (Bio-Rad). The calculated threshold cycle (Ct) corresponding to a target gene was normalized by the Ct of the control gyrB gene. The topoisomerase gyrB gene was chosen as a control because it showed no significant variation of gyrB expression. Experiments were performed in triplicate. The sequences of primers used in the quantitative reverse transcription-PCR (qRT-PCR) analysis are listed in Supplementary Table S5.
Propanediol utilization assay
A neutral-red pH indicator was used to test for the formation of propionate61. The color of this indicator changes from red to yellow between pH 6.8 and 8.0. When the neutral-red was added to the LB media (0.0033% as a final concentration), the use of 1,2-PDL produced a red color due to the reduction in pH, indicating the formation of propionate. Broth colors were examined after 8 h incubation in LB media.
β-Galactosidase assay
A Salmonella strain containing a lacZ gene was grown in LB medium with the indicated additives. β-Galactosidase assays were carried out in triplicate, and the activity was determined as described previously62.
Western blot analysis
Proteins were separated by molecular weight using SDS-PAGE and were transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 0.5% nonfat dry milk in 1× Tris-buffered saline-Tween 20 buffer and probed with anti-FLAG antibody (1:1,000 dilution, Sigma-Aldrich, Taufkirchen, Germany), anti-HA antibody (3:2,000 dilution, Sigma-Aldrich), or anti-DnaK antibody (1:10,000 dilution, Enzo Life Science, NY, USA) as primary antibodies. Anti-mouse IgG conjugated with peroxidase (3:5,000 dilution, Santa Cruz Biotechnology, CA, USA) was used as the secondary antibody in all western blot experiments. The chemiluminescent signals were developed with the West-Zol plus western blot detection system (iNtRON Biotechnology, Seongnam, Korea).
Analysis of EIIANtr and HilD stability
Protein stability was determined using a previously described method63. Salmonella strains encoding EIIANtr-FLAG or HilD-HA protein from the chromosome were grown in LB medium with the indicated additives for 4 h, and chloramphenicol was added at 0.2 mg/ml to block de novo protein synthesis. Aliquots of those cultures were collected at the indicated time points after the addition of chloramphenicol and were subjected to western blotting, as described above.
Gentamicin protection assay
Caco-2 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (50 U/ml), and streptomycin (50 U/ml). At least 1 h before bacterial infection, a monolayer of 2.5 × 105 Caco-2 cells was prepared in a 24-well tissue culture plate and incubated in DMEM-10% FBS without antibiotics at 37 °C under 5% CO2. Bacteria were subcultured from an overnight culture (1:100) in fresh LB medium containing the indicated additives and grown for 4 h at 37 °C with aeration. Bacteria were applied to the cell monolayer at a multiplicity of infection (MOI) of 10. After 30 min of incubation at 37 °C under 5% CO2, non-invasive bacteria were removed by washing three times with pre-warmed phosphate-buffered saline (PBS) and were then incubated for 1 h with the pre-warmed medium supplemented with 100 μg/ml of gentamicin to kill extracellular bacteria. Afterward, the wells were washed three times with pre-warmed PBS, lysed in 1% Triton X-100 in PBS for 30 min at 37 °C, and then diluted in PBS. A dilution of the suspension was plated on LB agar medium to enumerate the CFU.
Additional Information
How to cite this article: Yoo, W. et al. Enzyme IIANtr regulates Salmonella invasion via 1,2-propanediol and propionate catabolism. Sci. Rep. 7, 44827; doi: 10.1038/srep44827 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by a grant (14162MFDS972) from the Ministry of Food and Drug Safety, Korea, in 2016 and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01053815).
Footnotes
The authors declare no competing financial interests.
Author Contributions W.Y., D.K., H.Y., and S.R. conceived of the study, participated in its design and coordination, and wrote the manuscript. W.Y. and D.K. performed all experiments.
References
- Powell B. S. et al. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J. Biol. Chem. 270, 4822–4839 (1995). [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Wang G. & Seok Y.-J. Parallel PTS systems. Arch. Biochem. Biophys. 453, 101–107 (2006). [DOI] [PubMed] [Google Scholar]
- Pflüger-Grau K. & Görke B. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Microbiol. 18, 205–214 (2010). [DOI] [PubMed] [Google Scholar]
- Rabus R., Reizer J., Paulsen I. & Saier M. H. Enzyme INtr from Escherichia coli A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J. Biol. Chem. 274, 26185–26191 (1999). [DOI] [PubMed] [Google Scholar]
- Zimmer B., Hillmann A. & Görke B. Requirements for the phosphorylation of the Escherichia coli EIIANtr protein in vivo. FEMS Microbiol. Lett. 286, 96–102 (2008). [DOI] [PubMed] [Google Scholar]
- Lee C.-R., Cho S.-H., Yoon M.-J., Peterkofsky A. & Seok Y.-J. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc. Natl. Acad. Sci. USA 104, 4124–4129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttmann D. et al. Stimulation of the potassium sensor KdpD kinase activity by interaction with the phosphotransferase protein IIANtr in Escherichia coli. Mol. Microbiol. 72, 978–994 (2009). [DOI] [PubMed] [Google Scholar]
- Lüttmann D., Göpel Y. & Görke B. The phosphotransferase protein EIIANtr modulates the phosphate starvation response through interaction with histidine kinase PhoR in Escherichia coli. Mol. Microbiol. 86, 96–110 (2012). [DOI] [PubMed] [Google Scholar]
- Lee C. R. et al. Potassium mediates Escherichia coli enzyme IIANtr‐dependent regulation of sigma factor selectivity. Mol. Microbiol. 78, 1468–1483 (2010). [DOI] [PubMed] [Google Scholar]
- Chavarría M., Kleijn R. J., Sauer U., Pflüger-Grau K. & de Lorenzo V. Regulatory tasks of the phosphoenolpyruvate-phosphotransferase system of Pseudomonas putida in central carbon metabolism. MBio 3, e00028–00012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn S., van Rijsewijk B. R. H., Sauer U. & Bettenbrock K. A role for EIIANtr in controlling fluxes in the central metabolism of E. coli K12. Biochim. Biophys. Acta 1833, 2879–2889 ( 2013). [DOI] [PubMed] [Google Scholar]
- Karstens K., Zschiedrich C. P., Bowien B., Stülke J. & Görke B. Phosphotransferase protein EIIANtr interacts with SpoT, a key enzyme of the stringent response, In Ralstonia eutropha H16. Microbiol. 160, 711–722 (2014). [DOI] [PubMed] [Google Scholar]
- Ronneau S., Petit K., De Bolle X. & Hallez R. Phosphotransferase-dependent accumulation of (p)ppGpp in response to glutamine deprivation in Caulobacter crescentus. Nat. Commun. 7, 11423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. et al. Salmonella pathogenicity island 2 expression negatively controlled by EIIANtr–SsrB interaction is required for Salmonella virulence. Proc. Natl. Acad. Sci. USA 107, 20506–20511 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa F. & Edelstein P. H. Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect. Immun. 69, 4782–4789 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. R. et al. Potassium mediates Escherichia coli enzyme IIA(Ntr) -dependent regulation of sigma factor selectivity. Mol. Microbiol. 78, 1468–1483, doi: 10.1111/j.1365-2958.2010.07419.x (2010). [DOI] [PubMed] [Google Scholar]
- Pflüger-Grau K., Chavarría M. & de Lorenzo V. The interplay of the EIIANtr component of the nitrogen-related phosphotransferase system (PTSNtr) of Pseudomonas putida with pyruvate dehydrogenase. Biochim. Biophys. Acta 1810, 995–1005 (2011). [DOI] [PubMed] [Google Scholar]
- Haraga A., Ohlson M. B. & Miller S. I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6, 53–66 (2008). [DOI] [PubMed] [Google Scholar]
- Ibarra J. A. & Steele‐Mortimer O. Salmonella–the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell. Microbiol. 11, 1579–1586 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staib L. & Fuchs T. M. From food to cell: nutrient exploitation strategies of enteropathogens. Microbiol. 160, 1020–1039 (2014). [DOI] [PubMed] [Google Scholar]
- Havemann G. D., Sampson E. M. & Bobik T. A. PduA is a shell protein of polyhedral organelles involved in coenzyme B12-dependent degradation of 1, 2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 184, 1253–1261 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson E. M. & Bobik T. A. Microcompartments for B12-dependent 1, 2-propanediol degradation provide protection from DNA and cellular damage by a reactive metabolic intermediate. J. Bacteriol. 190, 2966–2971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G., Gibson G. & Cummings J. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72, 57–64 (1992). [DOI] [PubMed] [Google Scholar]
- Hammelman T. et al. Identification of a new prp locus required for propionate catabolism in Salmonella Typhimurium LT2. FEMS Microbiol. Lett. 137, 233–239 (1996). [DOI] [PubMed] [Google Scholar]
- Horswill A. R. & Escalante-Semerena J. C. Propionate catabolism in Salmonella Typhimurium LT2: two divergently transcribed units comprise the prp locus at 8.5 centisomes, prpR encodes a member of the sigma-54 family of activators, and the prpBCDE genes constitute an operon. J. Bacteriol. 179, 928–940 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios S., Starai V. J. & Escalante-Semerena J. C. Propionyl coenzyme A is a common intermediate in the 1, 2-propanediol and propionate catabolic pathways needed for expression of the prpBCDE operon during growth of Salmonella enterica on 1, 2-propanediol. J. Bacteriol. 185, 2802–2810 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C. C. et al. The intestinal fatty acid propionate inhibits Salmonella invasion through the post‐translational control of HilD. Mol. Microbiol. 87, 1045–1060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach R. G. et al. Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell. Microbiol. 10, 2364–2376, doi: 10.1111/j.1462-5822.2008.01218.x (2008). [DOI] [PubMed] [Google Scholar]
- Main-Hester K. L., Colpitts K. M., Thomas G. A., Fang F. C. & Libby S. J. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infect. Immun. 76, 1024–1035, doi: 10.1128/IAI.01224-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases I., Lopez J.-A., Albar J.-P. & De Lorenzo V. c. Evidence of multiple regulatory functions for the PtsN (IIANtr) protein of Pseudomonas putida. J. Bacteriol. 183, 1032–1037 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov R. L., Koonin E. V. & Lipman D. J. A genomic perspective on protein families. Science 278, 631–637 (1997). [DOI] [PubMed] [Google Scholar]
- Lee C. R. et al. Requirement of the dephospho‐form of enzyme IIANtr for derepression of Escherichia coli K‐12 ilvBN expression. Mol. Microbiol. 58, 334–344 (2005). [DOI] [PubMed] [Google Scholar]
- Lee C. R. et al. Reciprocal regulation of the autophosphorylation of enzyme INtr by glutamine and α‐ketoglutarate in Escherichia coli. Mol. Microbiol. 88, 473–485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier C. D. & Slauch J. M. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 185, 5096–5108 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. M., Bajaj V. & Lee C. A. A 40 kb chromosomal fragment encoding Salmonella Typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K‐12 chromosome. Mol. Microbiol. 15, 749–759 (1995). [DOI] [PubMed] [Google Scholar]
- Doucette C. D., Schwab D. J., Wingreen N. S. & Rabinowitz J. D. α-Ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat. Chem. Biol. 7, 894–901 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo W. et al. Fine-tuning of amino sugar homeostasis by EIIANtr in Salmonella Typhimurium. Sci. Rep. 6, 33055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimova G., Ullmann A. & Ladant D. A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol. 328, 59–73 (2000). [DOI] [PubMed] [Google Scholar]
- Ailion M., Bobik T. A. & Roth J. R. Two global regulatory systems (Crp and Arc) control the cobalamin/propanediol regulon of Salmonella Typhimurium. J. Bacteriol. 175, 7200–7208 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Newman J. D. & Keasling J. D. Catabolite repression of the propionate catabolic genes in Escherichia coli and Salmonella enterica: evidence for involvement of the cyclic AMP receptor protein. J. Bacteriol. 187, 2793–2800 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis R. M., Adams L. G., Ficht T. A. & Bäumler A. J. Contribution of Salmonella Typhimuriumvirulence factors to diarrheal disease in calves. Infect. Immun. 67, 4879–4885 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. et al. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71, 1–12 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 20, 263–271 (1996). [DOI] [PubMed] [Google Scholar]
- Ochman H., Soncini F. C., Solomon F. & Groisman E. A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93, 7800–7804 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka W. T. & Zhang Q. Phenotypic and genotypic evidence for L-fucose utilization by Campylobacter jejuni. J. Bacteriol. 193, 1065–1075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt N. et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. Isme J. 8, 1323–1335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chávez F. & Bäumler A. J. The pyromaniac inside you: Salmonella metabolism in the host gut. Annu. Rev. Microbiol. 69, 31–48 (2015). [DOI] [PubMed] [Google Scholar]
- Jakobson C. M. & Tullman-Ercek D. Dumpster diving in the gut: bacterial microcompartments as part of a host-associated lifestyle. PLoS Pathog. 12, e1005558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Lawrence J. & Bobik T. Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol. 50, 137–181 (1996). [DOI] [PubMed] [Google Scholar]
- Winter S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley T. D. et al. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect. Immun. 76, 403–416 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B. et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5, e244 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M. Salmonella pathogenicity island 2. Mol. Microbiol. 36, 1015–1023 (2000). [DOI] [PubMed] [Google Scholar]
- Nuccio S.-P. & Bäumler A. J. Comparative analysis of Salmonella genomes identifies a metabolic network for escalating growth in the inflamed gut. MBio 5, e00929–00914 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A. & Wanner B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 97, 6640–6645, doi: 10.1073/pnas.120163297 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncini F. C., Véscovi E. G. & Groisman E. A. Transcriptional autoregulation of the Salmonella Typhimurium phoPQ operon. J. Bacteriol. 177, 4364–4371 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi M., Ellermeier C. D., Slauch J. M. & Gunn J. S. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J. Bacteriol. 187, 7407–7416 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N. L. & Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella Typhimurium. Methods Enzymol. 204, 18–43 (1991). [DOI] [PubMed] [Google Scholar]
- Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A., Williams B. A., McCue K., Schaeffer L. & Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008). [DOI] [PubMed] [Google Scholar]
- Parsons J. B. et al. Biochemical and structural insights into bacterial organelle form and biogenesis. J. Biol. Chem. 283, 14366–14375 (2008). [DOI] [PubMed] [Google Scholar]
- Miller J. H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. (Cold Spring Harbor Laboratory, 1992). [Google Scholar]
- Biran D., Gur E., Gollan L. & Ron E. Z. Control of methionine biosynthesis in Escherichia coli by proteolysis. Mol. Microbiol. 37, 1436–1443 (2000) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.