Abstract
Advancement of RNAi-based therapeutics depends on effective delivery to the site of protein synthesis. Although intravenously administered, multi-component delivery vehicles have enabled small interfering RNA (siRNA) delivery and progression into clinical development, advances of single-component, systemic siRNA delivery have been challenging. In pre-clinical models, attachment of a triantennary N-acetylgalactosamine (GalNAc) ligand to an siRNA mediates hepatocyte uptake via the asialoglycoprotein receptor enabling RNAi-mediated gene silencing. In this phase 1 study, we assessed translation of this delivery approach by evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of a GalNAc-siRNA conjugate, revusiran, targeting transthyretin (TTR). Subjects received a placebo or ascending doses of revusiran subcutaneously ranging from 1.25–10 mg/kg in the single and 2.5–10 mg/kg in the multiple ascending dose phases. Revusiran was generally well tolerated, with transient, mild to moderate injection site reactions the most common treatment-emergent adverse events. Doses of 2.5–10 mg/kg revusiran elicited a significant reduction of serum TTR versus the placebo (p < 0.01), with mean TTR reductions of approximately 90% observed with multiple dosing. These results demonstrate translation of this novel delivery platform, enabling clinical development of subcutaneously administered GalNAc-siRNAs for liver-based diseases.
Keywords: RNAi, GalNAc-siRNA, asialoglycoprotein receptor, revusiran
This phase I study of revusiran demonstrated proof of concept for a subcutaneously administered siRNA that utilizes an N-acetylgalactosamine (GalNAc) ligand for hepatocyte-specific delivery. These results enabled clinical development of siRNA-GalNAc conjugates for treatment of liver-derived diseases and supported adoption of this delivery approach for other oligonucleotide-based therapeutics, including antisense oligonucleotides and anti-microRNAs.
Introduction
RNAi is an evolving approach for the potential treatment of genetic, metabolic, infectious, and malignant disease through its ability to selectively suppress disease-causing genes. The utility of this approach relies on productive delivery of the small interfering RNA (siRNA) to the site of protein synthesis, where the RNA-induced silencing complex (RISC) resides.1, 2 Initial advances in siRNA delivery were made through the use of intravenously (i.v.) administered, multi-component delivery systems such as lipid nanoparticles (LNPs) and polymers, resulting in a clinical proof of concept for systemic RNAi.3, 4 Although clinical progress has been made with these multi-component systems, they are encumbered by the need for i.v. administration and, in the case of LNPs, pre-medication with steroids to mitigate infusion-related reactions.3 As such, development of a single-component siRNA delivery platform amenable to subcutaneous (s.c.) administration is an attractive approach.
The incorporation of a ligand to facilitate uptake via a cell surface receptor has been explored for efficient drug delivery. Because of its abundant expression on hepatocytes, ability to support multiple rounds of uptake, and ligand specificity, the asialoglycoprotein receptor (ASGPR) has been utilized for liver-specific drug and gene delivery in animals5 and for delivery of radiopharmaceuticals to hepatocytes in humans.6 More recently, attachment of a N-acetylgalactosamine (GalNAc) ligand directly to an siRNA has been shown to mediate uptake into hepatocytes, resulting in potent and durable target mRNA knockdown in pre-clinical species (Figure 1).7
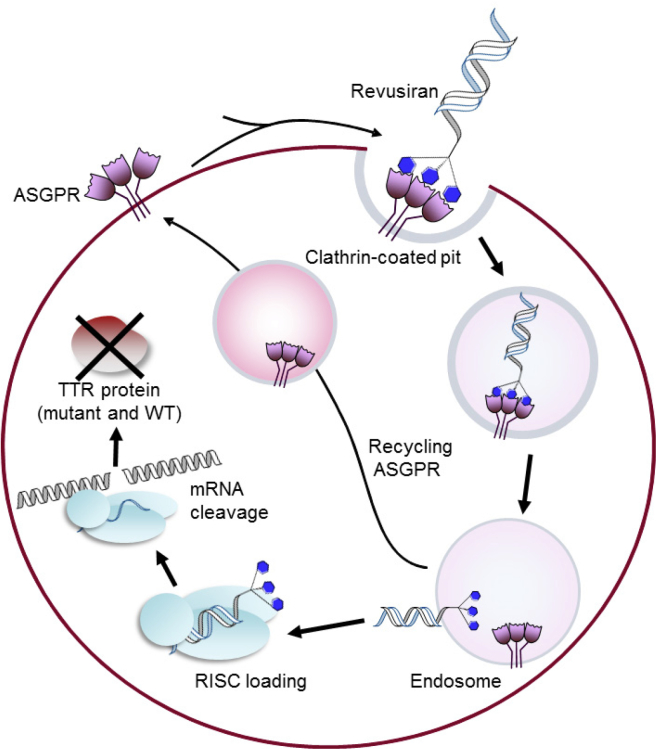
Figure 1.
Mechanism of Hepatocyte Uptake and Action of Revusiran
The siRNA targeting TTR mRNA is conjugated with a triantennary GalNAc. GalNAc binds to the ASGPR, which is highly expressed on hepatocytes, thus targeting revusiran to the liver. The revusiran-ASGPR complex is then taken into hepatocytes by clathrin-mediated endocytosis, where the siRNA causes TTR mRNA destruction through the RISC in the cytoplasm. The ASGPR is recycled to the cell surface for multiple rounds of siRNA uptake.
Revusiran is an investigational RNAi therapeutic agent for the treatment of transthyretin (TTR)-mediated amyloidosis (ATTR amyloidosis), a rare, multisystemic disease affecting ∼50,000 people worldwide, resulting from the deposition of insoluble TTR amyloid fibrils in various organs and tissues.8, 9 Autosomal dominant mutations of the TTR gene destabilize the liver-expressed native tetramer, yielding amyloidogenic TTR monomers and oligomers in circulation that form amyloid deposits in the heart, peripheral nerves, and gastrointestinal tract, leading to either cardiomyopathy and/or polyneuropathy. More than 100 different TTR mutations leading to hereditary ATTR amyloidosis (h-ATTR amyloidosis) have been described, and clinical presentation varies according to the TTR gene mutation.10, 11 In addition to the inherited form of the disease, deposition of wild-type (WT) TTR in the heart can also occur, leading to cardiomyopathy.12 Regardless of the form of ATTR amyloidosis, disease progression following symptom onset is associated with a substantial deterioration in patients’ quality of life and, ultimately, leads to death within approximately 5–15 years of diagnosis.8, 12
Revusiran is comprised of a 2′-deoxy-2′-fluoro- and 2′-O-methyl-containing siRNA directed against a region of the human TTR mRNA shown to be conserved in WT and all documented variants of the TTR gene, conjugated to GalNAc, and is amenable to subcutaneous delivery.7, 13 This hepatocyte-selective GalNAc-mediated delivery approach is well suited to targeting TTR, given that the majority of circulating TTR (>95%) is derived from hepatocytes.14 Potent, specific, and durable RNAi-mediated TTR suppression has been demonstrated in rodents and non-human primates (NHPs; cynomolgus monkeys) by employing this GalNAc-siRNA platform;7, 13 however, translation of this approach in humans had not been demonstrated.
Here, we report the first ever clinical translation of this GalNAc-siRNA delivery platform in a phase 1 trial of revusiran. We assessed the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single and multiple doses of revusiran in a phase 1 healthy volunteer trial designed to determine the activity and optimal dosing regimen for subsequent trials in patients with ATTR amyloidosis.
Results
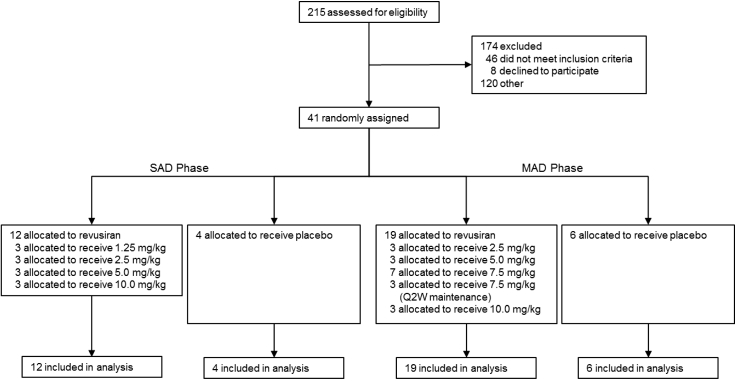
These data describe the weight-based dose escalation part of this randomized, double-blind, placebo-controlled phase 1 trial in healthy volunteers. A total of 41 subjects were recruited across the single ascending dose (SAD) and multiple ascending dose (MAD) phases of the study from February 25, 2013 to August 29, 2014. Twelve subjects were randomly assigned to receive revusiran and four to receive a placebo in the SAD phase (Figure 2). In the MAD phase, 19 subjects received revusiran (three subjects in each of the 2.5, 5, and 7.5 mg/kg once every 2 weeks [Q2W] and 10 mg/kg cohorts and seven in the 7.5 mg/kg once weekly [QW] cohort), and six received a placebo (Figure 2). Overall, 39 subjects (95%) completed the study; two subjects withdrew consent (one each in the 1.25 mg/kg SAD and 7.5 mg/kg QW MAD cohorts). All 41 study participants were included in the data analyses. The baseline characteristics for all study participants are listed in Table 1.
Figure 2.
Trial Profile
SAD dose cohorts preceded enrollment of the MAD dose cohorts.
Table 1.
Demographics and Baseline Characteristics
| Characteristic | SAD Phase |
MAD Phase |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 4)a | Revusiran (mg/kg) |
Placebo (n = 6) | Revusiran (mg/kg) |
||||||||
| 1.25 (n = 3) | 2.5 (n = 3) | 5.0 (n = 3) | 10.0 (n = 3) | 2.5 QW (n = 3) | 5.0 QW (n = 3) | 7.5 QW (n = 7) | 7.5 Q2W (n = 3) | 10.0 QW (n = 3) | |||
| Median age (years) (range) | 45.5 (40–53) | 25.0 (23–43) | 25.0 (20–48) | 27.0 (24–29) | 36.0 (33–47) | 44.5 (22–54) | 38.0 (30–51) | 26.0 (21–41) | 47.0 (23–55) | 41.0 (36–42) | 23.0 (21–23) |
| Male sex (n) (%) | 4 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 6 (100) | 3 (100) | 3 (100) | 6 (86) | 3 (100) | 3 (100) |
| Mean weight (kg) (SD) | 82.6 (13.6) | 74.0 (5.6) | 91.3 (12.8) | 81.8 (19.7) | 89.2 (12.9) | 75.7 (10.7) | 72.5 (12.6) | 83.0 (6.3) | 84.5 (9.0) | 81.5 (14.8) | 74.7 (10.7) |
| Mean TTR (μg/mL) (SD) | 322.1 (58.1) | 266.4 (19.3) | 259.5 (12.0) | 334.1 (19.6) | 290.6 (44.7) | 258.4 (42.7) | 227.6 (60.1) | 235.7 (26.3) | 280.6 (24.2) | 283.1 (9.9) | 254.6 (37.3) |
The SAD placebo cohort contained one subject of Hispanic or Latino ethnicity. All other subjects were non-Hispanic or non-Latino.
Single and multiple doses of revusiran were generally well tolerated, with no dose-limiting toxicities (DLTs), flu-like symptoms, serious adverse events (SAEs), or treatment discontinuations because of treatment-emergent adverse events (TEAEs). The majority of TEAEs were of mild to moderate intensity. Severe events were reported in only two subjects. One case of syncope occurred in the 5.0 mg/kg SAD cohort that was deemed unrelated to the study drug, and one case of an injection site reaction (ISR) that was considered severe (erythema > 10 cm) occurred in a subject in the 7.5 mg/kg QW cohort in the MAD phase. The most commonly observed TEAEs were mild to moderate ISRs (defined as two or more mild signs or symptoms or one moderate or severe sign or symptom; Table S1); these were only reported in subjects who received multiple doses of revusiran (13 of 19 subjects; Table 2; Table S2). The most frequently reported ISR signs and symptoms were transient erythema (47.4%), pain (31.6%), and swelling (36.8%) that self-resolved. Aside from events associated with the injection site, headache was the only TEAE observed in more than 10% of subjects receiving revusiran as well as the placebo. There were no clinically relevant abnormalities recorded for renal function (creatinine), hematological parameters including platelets, thyroid function tests, C-reactive protein (CRP), cytokines, or urinalysis during either phase of the study (data not shown). Mild, transient, asymptomatic elevations in aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) (≤2× upper limit of normal [ULN]) were observed in 9 of 19 subjects who received multiple doses of revusiran and 1 of 6 subjects who received the placebo (Figure S1). These transaminase elevations were not clinically significant, did not concur with elevations in total bilirubin and alkaline phosphatase (ALP), and showed no obvious dose relationship. All subjects with transaminase elevations were recovering toward baseline either during treatment or within several weeks after the treatment was completed (Figure S1). Anti-drug antibodies were not observed after multiple dosing with revusiran (data not shown).
Table 2.
Safety and Tolerability: TEAEs Related to the Study Drug Occurring in ≥10% Subjects in Either the SAD or MAD Phases
| TEAE | SAD Phase |
MAD Phase |
All Revusiran (n = 31) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 4) | Revusiran (mg/kg) |
Placebo (n = 6) | Revusiran (mg/kg) |
|||||||||
| 1.25 (n = 3) | 2.5 (n = 3) | 5.0 (n = 3) | 10.0 (n = 3) | 2.5 QW (n = 3) | 5.0 QW (n = 3) | 7.5 QW (n = 7)a | 7.5 Q2W (n = 3) | 10.0 QW (n = 3) | ||||
| Injection site reactionb | 0 | 0 | 0 | 0 | 0 | 1 (17) | 1 (33) | 1 (33) | 5 (71) | 3 (100) | 3 (100) | 13 (42) |
| Injection site erythema | 0 | 0 | 0 | 0 | 0 | 0 | 2 (67) | 1 (33) | 2 (29) | 3 (100) | 2 (67) | 10 (53) |
| Injection site pain | 0 | 0 | 0 | 0 | 1 (33) | 0 | 0 | 0 | 2 (29) | 2 (67) | 0 | 5 (16) |
| Headache | 1 (25) | 0 | 1 (33) | 1 (33) | 0 | 2 (33) | 0 | 0 | 2 (29) | 0 | 1 (33) | 5 (16) |
| Injection site swelling | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (43) | 0 | 0 | 3 (10) |
One subject in the 7.5 mg/kg group was excluded from this analysis because only one post-dose data point was available.
An injection site reaction was defined as two or more mild signs or symptoms or one moderate or severe sign or symptom (Table S4).
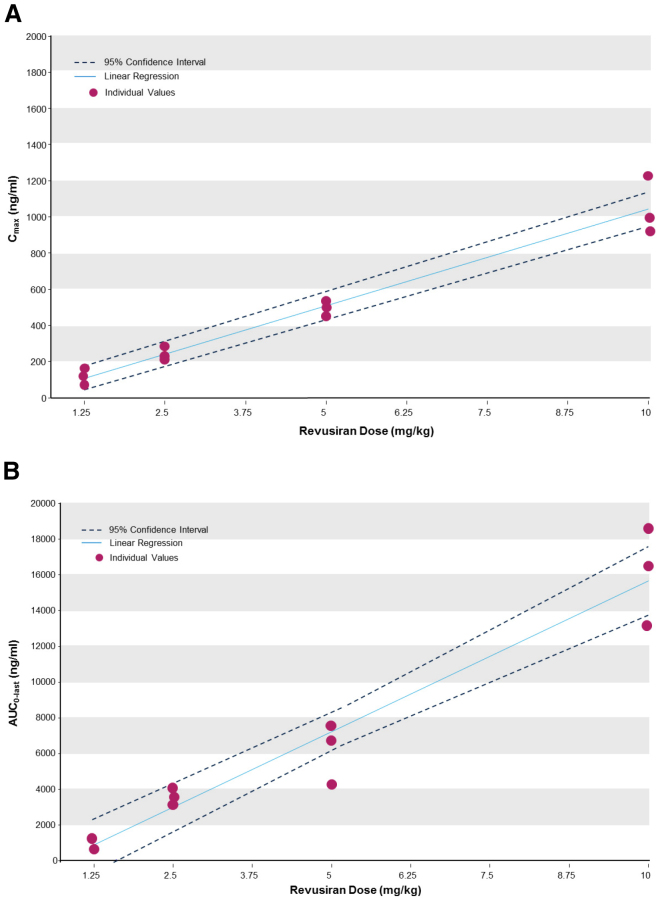
The plasma exposure of subcutaneously administered revusiran increased in a dose-proportional manner (Figure 3; Table S3) over the dose range tested. Maximum revusiran plasma concentrations were achieved between 2 and 6 hr post-administration, and the mean elimination half-life was typically in the 6- to 10-hr range. Importantly, no plasma accumulation was observed after multiple doses of revusiran, and PK parameters at each dose level were generally similar for single and multiple doses of revusiran (Table S3).
Figure 3.
Proportionality of Revusiran Plasma Cmax and AUC0–last after a Single Administration of Revusiran
(A and B) Proportionality of revusiran plasma observed maximum plasma concentration (Cmax) (A) and area under the plasma concentration-time curve from zero to the last measurable time point (AUC0–last) (B) after a single administration of revusiran.
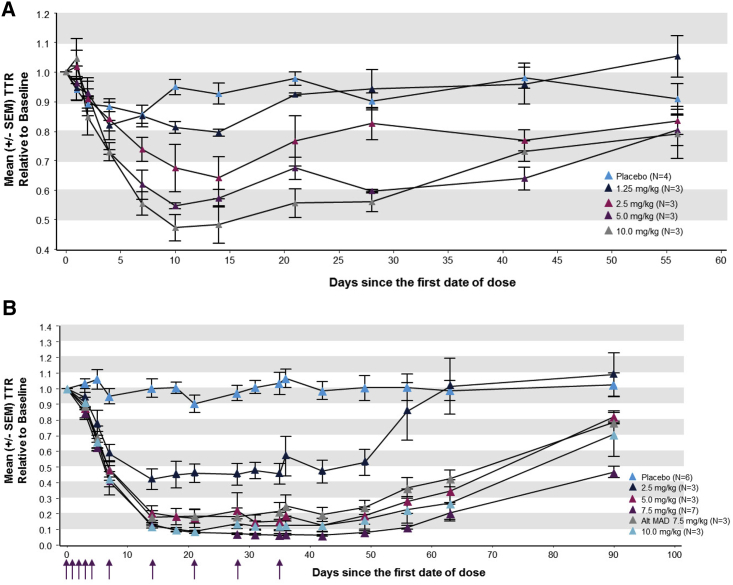
Subcutaneous administration of revusiran led to a significant reduction in serum TTR versus placebo at single doses of 2.5, 5, and 10 mg/kg by day 10 (p < 0.05; analysis of covariance [ANCOVA] model) and maximum TTR reductions achieved between days 10 and 14 (Figure 4A). The extent of TTR knockdown increased in a dose-related manner, with the greatest mean (SD) reduction in TTR of 53.3% (8.1%) and maximum knockdown in an individual subject of 59.6% after a single subcutaneous dose of 10 mg/kg revusiran (Table 3).
Figure 4.
Pharmacodynamic Effect of Revusiran on Serum TTR Levels
(A and B) Mean (± SEM) change in serum TTR over time relative to baseline in the SAD (A) and MAD (B) phase. Arrows indicate days of dosing in the multidose cohorts at 2.5, 5, and 7.5 (QW) and 10 mg/kg revusiran (days 0–4 and then weekly from days 7–35). The alternate MAD 7.5 mg/kg cohort was dosed on days 0–4 and then every other week from days 7–35.
Table 3.
Mean Maximal Knockdown and Individual Maximum Knockdown for TTR per Dose Cohort Relative to Baseline values
| KD | SAD Phase |
MAD Phase |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 4) | Revusiran (mg/kg) |
Placebo (n = 6) | Revusiran (mg/kg) |
||||||||
| 1.25 (n = 3) | 2.5 (n = 3) | 5 (n = 3) | 10 (n = 3) | 2.5 QW (n = 3) | 5 QW (n = 3) | 7.5 QW (n = 7) | 7.5 Q2W (n = 3) | 10 QW (n = 3) | |||
| Maximum TTR KD (%) | 22.0 | 22.7 | 49.0a | 47.7a | 59.6a | 33.4 | 70.6b | 93.3b | 96.2b | 91.1b | 94.0b |
| TTR KD at nadir (mean ± SD) (%) | 18.3 ± 4.19 | 21.6 ± 1.19 | 37.7 ± 12.33 | 46.7 ± 0.88 | 53.3 ± 8.11 | 16.9 ± 10.49 | 58.2 ± 11.12 | 87.5 ± 7.22 | 87.9 ± 16.77 | 83.4 ± 7.27 | 92.4 ± 1.50 |
KD, knockdown.
Significant reduction versus placebo (p <0.05).
Significant reduction versus placebo (p <0.01).
A significant reduction in TTR versus placebo (p <0.01, ANCOVA model) was also observed across all dose cohorts in the MAD phase (Table 3). For all dose cohorts, mean TTR levels reached a nadir on day ≥14 and were maintained during weekly revusiran dosing through approximately 2–3 weeks after dosing cessation before TTR levels began to recover (Figure 4B). The mean (SD) maximal TTR reduction from baseline for weekly revusiran ≥5 mg/kg ranged from 87.5% (7.22) to 92.4% (1.50) (Table 3). In comparison with weekly dosing, the mean (SD) maximal TTR reduction was slightly lower when revusiran (7.5 mg/kg) was dosed Q2W (Table 3). Across individual subjects, the maximum reduction in TTR was 96.2%, achieved in the 7.5 mg/kg QW cohort (Table 3); similar levels of robust TTR reduction were observed in the revusiran 5.0, 7.5, and 10 mg/kg weekly dosing cohorts (Table 3). Multiple dosing of revusiran was also tested in NHPs, and comparison with these data revealed a strong association between TTR knockdown in humans and NHPs (R2 = 0.83, p < 0.001; Figure S2), demonstrating clinical translation of this subcutaneous GalNAc-siRNA delivery platform.
Consistent with TTR’s role in vitamin A transport, reductions in retinol binding protein (RBP) and vitamin A were also observed in the revusiran dosing cohorts (Table S4). Mean maximal reductions of 68.4%–70.6% and 83.7%–85.6% for RBP and vitamin A, respectively, were achieved in the MAD phase with weekly revusiran ≥5 mg/kg. These reductions in RBP and vitamin A were strongly associated with the reductions observed in serum TTR (R2 = 0.87 and 0.94 for RBP and vitamin A, respectively, in the MAD cohorts; both p < 10−15; Figure S3).
Discussion
The success of RNAi-based therapies will hinge on the ability to deliver siRNA to the target cell and site of RNAi action, resulting in RISC-mediated target mRNA cleavage and inhibition of protein synthesis. Here we provide the first demonstration in humans of the safety and pharmacodynamic activity of a GalNAc-siRNA conjugate that effectively targets hepatocytes and mediates knockdown of liver-expressed TTR, the pathogenic protein of ATTR amyloidosis. Single and multiple subcutaneous doses of revusiran were generally well tolerated and resulted in potent and durable TTR knockdown in healthy volunteers, demonstrating clinical translation of this novel GalNAc-siRNA delivery platform.
Single subcutaneous doses of revusiran in humans resulted in a dose-related increase in TTR knockdown, with a mean maximal reduction of >50% achieved with administration of 10 mg/kg. Multiple dosing of revusiran resulted in rapid mean maximal TTR reduction of >90%, which was maintained with weekly doses of ≥5 mg/kg. The reduction in TTR was still apparent with revusiran ≥5 mg/kg at least 7 weeks after cessation of dosing, demonstrating the lasting effect of this treatment. The strong correlation between human and NHP TTR knockdown suggests that NHPs are predictive of human PD for GalNAc-siRNA conjugates and provides a valuable preclinical model for translational research of other GalNAc-conjugated siRNA-based therapeutic agents.
The GalNAc-siRNA conjugate is the first single-component, subcutaneously administered delivery approach for targeting siRNA to the liver. Prior to this advancement, successful RNAi-mediated TTR knockdown in humans had been demonstrated with intravenously administered TTR siRNA encapsulated in a multi-component LNP (patisiran) co-administered with steroid pre-medication to mitigate the risk of infusion-related reactions.3 These LNPs are bound by apolipoprotein E and taken up by low-density lipoprotein (LDL) receptors expressed on hepatocytes, where they are internalized by endocytosis.15 In contrast, subcutaneously administered revusiran is targeted to the liver by a simple carbohydrate ligand that enables receptor-mediated uptake by the ASGPR expressed on hepatocytes and does not require any pre-medication. The level of TTR knockdown observed with revusiran administration in this study is comparable with that reported for intravenous patisiran (0.3 mg/kg every 3 weeks16) and greater than that reported for the antisense oligonucleotide (ASO) agent IONIS-TTRRX in healthy volunteers.17, 18
The most common TEAEs for revusiran were mild to moderate ISRs and the signs and symptoms associated with these reactions. These events were transient and resolved without any intervention. Injection site events are not uncommon with subcutaneous drug administration and are among the most frequently reported AEs following dosing of other oligonucleotide-based therapeutic agents.19 Importantly, there was no evidence of systemic immune stimulation, with no relevant changes in serum levels of CRP or cytokines and no evidence of anti-drug antibody production with revusiran treatment. Furthermore, no clinically relevant changes were observed in hepatic, renal, hematological, or thyroid function tests with revusiran administration.
Because revusiran targets a region within the TTR mRNA that is common to both WT and all documented TTR mutations, it is expected that this GalNAc-siRNA will lower the levels of all forms of TTR involved in ATTR amyloidosis. The reduction of both WT and mutant TTR is hypothesized to prevent further formation of amyloid deposits and potentially promote their regression. Elimination of mutant TTR is the fundamental driver behind liver transplantation for ATTR amyloidosis with polyneuropathy, and the clinical benefits associated with this procedure highlight the potential effect of therapies that can lower hepatic TTR.20, 21 Indeed, the high level of TTR knockdown achieved with revusiran (∼90%) is comparable with the reduction in mutant TTR following liver transplantation, with the added advantage of affecting both mutant and WT TTR production. The high potency of revusiran is also particularly promising because a reduction in levels of amyloidogenic protein of only 50% has been associated with clinical benefits in light-chain amyloidosis.22 The activity of revusiran described here demonstrates the capacity for GalNAc-siRNA to effectively target the liver, where TTR is predominantly produced, providing a therapeutic strategy for ATTR amyloidosis.
The utility of this GalNAc conjugate approach for efficient and potent hepatocellular siRNA delivery has provided a robust platform for the development of RNAi-based therapies in liver-based diseases, with GalNAc-siRNA conjugates in development for the treatment of hemophilia,23 acute hepatic porphyria,24 and hepatic infectious diseases,25 among others. Indeed, GalNAc-mediated delivery has been deployed with other oligonucleotide-based therapeutic agents, including ASOs26 and anti-microRNAs (miRNAs),27 as demonstrated in preclinical studies and their advancement into clinical development. The data presented in this study provide proof of concept for human translation of this GalNAc-siRNA platform, thus promoting the clinical adoption of this approach for future therapies.
Materials and Methods
Study Design and Participants
This phase 1, randomized, double-blind, placebo-controlled study was conducted at two centers in the United Kingdom (Covance Clinical Research Unit, Leeds and Hammersmith Medicines Research). Eligible subjects were healthy Caucasian volunteers aged 18–55 years with a body mass index of 18.0–30 kg/m2. Subjects had ALT, AST, and gamma-glutamyl transpeptidase levels of <1.5× ULN, albumin ≥3.9 g/dL, and total bilirubin and ALP levels that were considered clinically normal by the investigator. Women were required to be postmenopausal or surgically sterilized; men had to use effective means of contraception. Subjects with hepatitis B or C or HIV infection were excluded.
This study was performed in accordance with the principles associated with the World Health Organization Declaration of Helsinki and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Written informed consent was obtained from all participating subjects. The clinical trial protocol was approved by an independent ethics committee. This trial is registered with ClinicalTrials.gov (NCT01814839).
Randomization and Masking
Assignment of treatment was masked from the study subjects, principal investigators, medical monitors, and study site personnel. Subjects were randomized to receive either revusiran or a placebo (normal saline) based on a cohort-specific randomization list generated by the research site’s biostatistician. Only the site pharmacist was aware of the treatment assignment.
Procedures
Four subjects were assessed at each dose level, with three receiving revusiran and one receiving the placebo in all SAD and MAD cohorts. For each cohort, dosing was staggered so that dosing of subjects 1 and 2 occurred no sooner than 48 hr apart. Dosing proceeded for the remaining subjects based on safety review committee (SRC) assessment of safety and tolerability and no sooner than 48 hr after subject 2. Dose escalation occurred sequentially in the SAD and MAD cohorts based on SRC review of safety data. Subjects in the SAD cohorts received a single subcutaneous dose of 1.25, 2.5, 5, or 10 mg/kg revusiran or placebo. Subjects in the MAD cohorts received a total of 10 subcutaneous doses of the study drug (one dose per day on days 0–4 and then weekly from days 7 to 35 [maintenance]) at 2.5, 5, and 10 mg/kg. Based on acceptable tolerability, additional MAD cohorts were added to investigate revusiran 7.5 mg/kg with either QW or Q2W maintenance dosing. Subjects were followed at screening (days –45 to –2), admission (day –1), dosing (up to day 35), and follow-up (up to 56 days and 90 days after the first dose in the SAD and MAD phases, respectively).
The primary objective was safety and tolerability of single and multiple doses of revusiran. Reported adverse events (AEs) were collected from the first dose until 28 days after the last dose and summarized by the Medical Dictionary for Regulatory Activities.28 AEs were classified as mild, moderate, or severe, and their potential relationship to the study drug was recorded. Treatment-emergent adverse events were defined as events that started after exposure to the study drug or worsened in severity after dosing. An ISR was defined as two or more mild signs or symptoms or one moderate or severe sign or symptom (Table S1). The severity of injection site signs and symptoms was based on a Food and Drug Administration (FDA) guidance document for vaccine trials in healthy volunteers.29 Clinical laboratory tests included hematology, serum chemistries, electrocardiograms, liver function tests, thyroid function parameters, coagulation, CRP, cytokines, and urinalysis. Anti-drug antibody production was assessed using a validated ELISA (Supplemental Materials and Methods).
The secondary objectives were to characterize the pharmacokinetics of revusiran and to assess its pharmacodynamic effect on serum TTR levels. In addition, the effect of revusiran on secondary markers of TTR lowering, vitamin A and RBP, was explored. Samples were collected at pre-specified times for these measures (Supplemental Materials and Methods). TTR concentration was evaluated using a validated ELISA (Charles River Laboratories). Biomnis Specialized Medical Pathology evaluated vitamin A using high-performance liquid chromatography (HPLC) and RBP by nephelometry.
Statistical Analysis
This study was not powered for formal hypothesis testing. Sample size was based on the planned dose escalation scheme. All subjects who received at least one dose of the study drug were included in the safety population; those who had at least one post-dose sample for PK and PD parameters were included in these populations, respectively. TTR knockdown at the nadir was calculated per individual subject as the lowest TTR level relative to baseline (average of the three pre-dose measures at screening and days –1 and 0) on any post-dose day. Analysis of variance models (with day and treatment group as factors) and ANCOVA models (with day and treatment group as factors and baseline TTR as a covariate) were used to assess TTR knockdown at the nadir and over time; Tukey’s post hoc tests examined pairwise comparisons (i.e., between dose groups at a particular time point or at the nadir). Linear regression was used to assess the correlations between TTR knockdown in human and NHPs and the correlations between TTR knockdown and reductions in circulating RBP and vitamin A. No substitutions were made for missing data points. Continuous variables were described using descriptive statistics, and categorical and ordinal variables were presented as frequencies and percentages. Analyses were conducted using SAS for Windows (version 9.2 or higher; SAS Institute) and/or R for Windows (version 3.0).
Author Contributions
Conceptualization, T.S.Z., V.K., A.C., R.H., B.R.B., S.N., A.V., and J.G.; Methodology, T.S.Z., V.K., A.C., R.H., B.R.B., J.C., M.B., S.N., A.V., and J.G.; Formal Analysis, R.H. and B.R.B; Investigation and Resources in the Conduct of the Clinical Trial, J.C. and M.B.; Writing – Original Draft, T.S.Z and J.G.; Writing – Review & Editing, T.S.Z., V.K., A.C., R.H., B.R.B., J.C., M.B., S.N., A.V., and J.G.; Supervision, A.V. and J.G.
Conflicts of Interest
The authors received editorial support from Adelphi Communications funded by Alnylam Pharmaceuticals. The contract organization Prometrika was funded by Alnylam Pharmaceuticals to conduct some of the statistical analysis. Tracy S. Zimmermann, Verena Karsten, Amy Chan, Renta Hutabarat, Brian R. Bettencourt, Saraswathy Nochur, Akshay Vaishnaw, and Jared Gollob are employees and shareholders of Alnylam Pharmaceuticals. Joseph Chiesa is an employee of Covance Clinical Research Unit. Malcolm Boyce is an employee of Hammersmith Medicines Research.
Acknowledgments
This Phase 1 study was sponsored by Alnylam Pharmaceuticals. We would like to thank Jamie Harrop, Richard Coles, and Sue Ellen White of Alnylam Pharmaceuticals, Inc. for their involvement in the management of this study. Special thanks also to Christine Powell of Alnylam Pharmaceuticals for providing support for data analysis and review.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, three figures, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2016.10.019.
Supplemental Information
References
- 1.Dykxhoorn D.M., Novina C.D., Sharp P.A. Killing the messenger: short RNAs that silence gene expression. Nat. Rev. Mol. Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 2.Elbashir S.M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 3.Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 4.Wooddell C.I., Rozema D.B., Hossbach M., John M., Hamilton H.L., Chu Q., Hegge J.O., Klein J.J., Wakefield D.H., Oropeza C.E. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol. Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J., Nantz M.H., Zern M.A. Targeting hepatocytes for drug and gene delivery: emerging novel approaches and applications. Front. Biosci. 2002;7:d717–d725. doi: 10.2741/A806. [DOI] [PubMed] [Google Scholar]
- 6.Kokudo N., Vera D.R., Makuuchi M. Clinical application of TcGSA. Nucl. Med. Biol. 2003;30:845–849. doi: 10.1016/s0969-8051(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 7.Nair J.K., Willoughby J.L., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 8.Ando Y., Coelho T., Berk J.L., Cruz M.W., Ericzon B.G., Ikeda S., Lewis W.D., Obici L., Planté-Bordeneuve V., Rapezzi C. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J. Rare Dis. 2013;8:31. doi: 10.1186/1750-1172-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou X., Aguilar M.I., Small D.H. Transthyretin and familial amyloidotic polyneuropathy. Recent progress in understanding the molecular mechanism of neurodegeneration. FEBS J. 2007;274:1637–1650. doi: 10.1111/j.1742-4658.2007.05712.x. [DOI] [PubMed] [Google Scholar]
- 10.Connors L.H., Lim A., Prokaeva T., Roskens V.A., Costello C.E. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 11.Sekijima Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J. Neurol. Neurosurg. Psychiatry. 2015;86:1036–1043. doi: 10.1136/jnnp-2014-308724. [DOI] [PubMed] [Google Scholar]
- 12.Ruberg F.L., Berk J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler J.S., Chan A., Costelha S., Fishman S., Willoughby J.L., Borland T.D., Milstein S., Foster D.J., Gonçalves P., Chen Q. Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis. Amyloid. 2016;23:109–118. doi: 10.3109/13506129.2016.1160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stangou A.J., Hawkins P.N. Liver transplantation in transthyretin-related familial amyloid polyneuropathy. Curr. Opin. Neurol. 2004;17:615–620. doi: 10.1097/00019052-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K.N., Jayaraman M., Rajeev K.G., Cantley W.L., Dorkin J.R. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suhr O.B., Coelho T., Buades J., Pouget J., Conceicao I., Berk J., Schmidt H., Waddington-Cruz M., Campistol J.M., Bettencourt B.R. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J. Rare Dis. 2015;10:109. doi: 10.1186/s13023-015-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann E.J., Guo S., Benson M.D., Booten S., Freier S., Hughes S.G., Kim T.W., Jesse Kwoh T., Matson J., Norris D. Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides. Amyloid. 2016;23:148–157. doi: 10.1080/13506129.2016.1191458. [DOI] [PubMed] [Google Scholar]
- 18.Ackermann E.J., Guo S., Booten S., Alvarado L., Benson M., Hughes S., Monia B.P. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19(Suppl 1):43–44. doi: 10.3109/13506129.2012.673140. [DOI] [PubMed] [Google Scholar]
- 19.van Meer L., Moerland M., Gallagher J., van Doorn M.B., Prens E.P., Cohen A.F., Rissmann R., Burggraaf J. Injection site reactions after subcutaneous oligonucleotide therapy. Br. J. Clin. Pharmacol. 2016;82:340–351. doi: 10.1111/bcp.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ericzon B.G., Wilczek H.E., Larsson M., Wijayatunga P., Stangou A., Pena J.R., Furtado E., Barroso E., Daniel J., Samuel D. Liver Transplantation for Hereditary Transthyretin Amyloidosis: After 20 Years Still the Best Therapeutic Alternative? Transplantation. 2015;99:1847–1854. doi: 10.1097/TP.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 21.Suhr O.B., Larsson M., Ericzon B.G., Wilczek H.E., FAPWTR's investigators Survival After Transplantation in Patients With Mutations Other Than Val30Met: Extracts From the FAP World Transplant Registry. Transplantation. 2016;100:373–381. doi: 10.1097/TP.0000000000001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachmann H.J., Gallimore R., Gillmore J.D., Carr-Smith H.D., Bradwell A.R., Pepys M.B., Hawkins P.N. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br. J. Haematol. 2003;122:78–84. doi: 10.1046/j.1365-2141.2003.04433.x. [DOI] [PubMed] [Google Scholar]
- 23.Sehgal A., Barros S., Ivanciu L., Cooley B., Qin J., Racie T., Hettinger J., Carioto M., Jiang Y., Brodsky J. An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat. Med. 2015;21:492–497. doi: 10.1038/nm.3847. [DOI] [PubMed] [Google Scholar]
- 24.Chan A., Liebow A., Yasuda M., Gan L., Racie T., Maier M., Kuchimanchi S., Foster D., Milstein S., Charisse K. Preclinical Development of a Subcutaneous ALAS1 RNAi Therapeutic for Treatment of Hepatic Porphyrias Using Circulating RNA Quantification. Mol. Ther. Nucleic Acids. 2015;4:e263. doi: 10.1038/mtna.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepp-Lorenzino L., Abrams M., Carayannopoulos L., Koser M., Ludmerer S., Charisse K.B. Poster Session 4: Hepatitis B Therapy. Hepatology. 2014;60:1088A–1128A. [Google Scholar]
- 26.Prakash T.P., Graham M.J., Yu J., Carty R., Low A., Chappell A., Schmidt K., Zhao C., Aghajan M., Murray H.F. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat B., Neben S., Tay J., Liu K., Chau N., Hogan D. RG-101, a GalNAC-conjugated anti-miR employing a unique mechanism of action by targeting host factor microRNA-122 (miR-122), demonstrates potent activity and reduction of HCV in preclinical studies. Hepatology. 2013;58 1393A–1393A. [Google Scholar]
- 28.US Department of Health and Human Services . National Cancer Institute; 2009. Common terminology criteria for adverse events (CTCAE) version 4.0. [Google Scholar]
- 29.Food and Drug Administration . Food and Drug Administration; 2007. Guidance for industry. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.