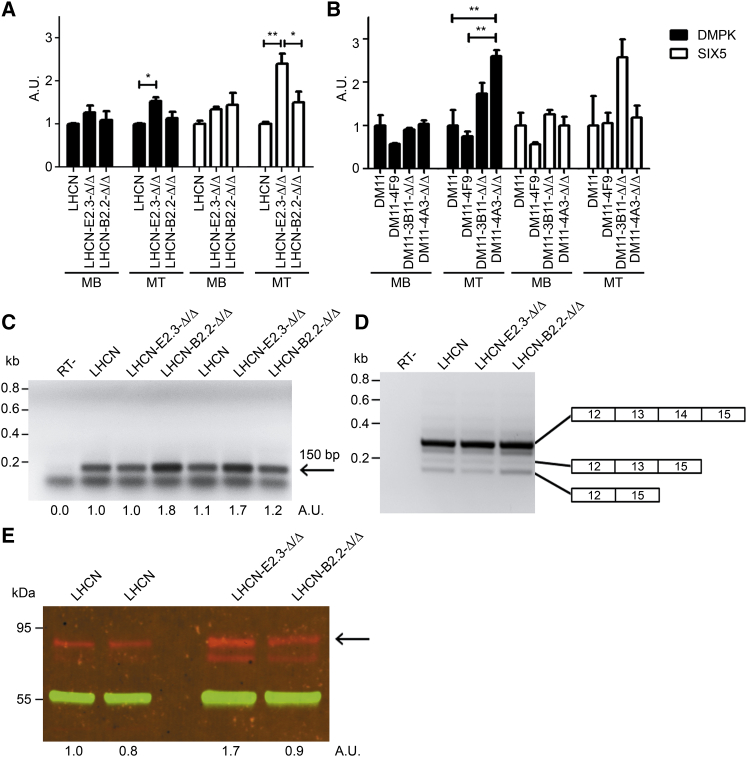
Figure 6.
Effects of (CTG⋅CAG)n Repeat Excision on Expression of RNA and Protein Products from Genes in the DM1 Locus
(A and B) RNA was isolated from (A) LHCN myoblasts (MBs) or LHCN- derivatives (see Table S4 for genotype specification) or myotubes (MTs) formed thereof after 5 days in differentiation medium or (B) from DM11 and CRISPR-edited derivative (see Table S4) MBs and MTs, and used for RT-qPCR analysis of expression of DMPK (black bars) and SIX5 (white bars). Bar heights in the diagram correspond to steady-state expression levels given in arbitrary units (n = 3; mean + SEM). (C) RT-PCR analysis of DM1-AS expression in myoblasts (signal strength of the specific 150-bp product is given in arbitrary units underneath; the lower band seen in all lanes represents a primer-dimer signal). (D) RT-PCR analysis of major splice isoforms of DMPK mRNA formed by alternative skipping of exon 13 to 14 or 14 regions in DMPK heterogeneous nuclear RNA (hnRNA) from myoblasts. (E) Visualization of DMPK protein production in parental and gene-edited LHCN myotubes (5 days of differentiation) by western blot analysis. The most abundant DMPK isoform, i.e., the protein produced from the longest RNA splice isoform with exons 13 to 14 included, has an apparent molecular weight of 80–85 kDa (arrow) and is present in all cells. The smaller DMPK isoform, lacking exon 13 to 14 sequences, comigrates with cross-reacting proteins.80 Variation in signal strength of immunostaining with polyvalent rabbit anti-DMPK antiserum (red) is given in arbitrary units below. Staining with monoclonal mouse-anti-β tubulin antibody (green) was used as control for loading and normalization.