Main Text
T cell therapies have shown promise against viral infections and malignancies. Moreover, advances in gene-editing technology hold the promise that T cell potency can be enhanced by gene modification, deletion, or addition.1 In this issue of Molecular Therapy, Hale et al.,2 in a single construct, combine CCR5 knockdown with a chimeric antigen receptor targeting the HIV envelope, a combination strategy that can potentially provide both anti-HIV activity independent of major histocompatibility complex (MHC) expression and protection of gene-modified T cells from HIV infection.
Exploitation of endogenous homology-directed recombination (HDR) pathways to introduce genetic modifications is a promising strategy to enhance T cell therapies. Harnessing HDR for gene modification involves the use of an exogenous DNA template to specify the exact outcome of DNA double-strand break repair. In other words, when a targeted DNA double-strand break is introduced, the HDR machinery can use exogenously provided single- or double-stranded DNA templates (which have sequence homology to the break site) to synthesize DNA that is used to repair the lesion, allowing for the precise insertion of gene products at the break site.3 For example, it was shown that such a mechanism may be utilized to specifically select a region where a transgene can be expressed (limiting insertional mutagenesis events) or, more intriguingly, to simultaneously disrupt a region expressing an unwanted gene and replace it with a foreign (or favorable) genetic construct.4
Disruption of unwanted functions in T cells has gained prominence recently in the cancer field, with encouraging clinical trials utilizing checkpoint inhibitors for the treatment of solid tumors and hematologic malignancies.5 The establishment of screening technologies seeking to identify additional genes involved in T cell suppression will only increase the number of potential immunotherapy targets for disruption.6 Besides editing out such checkpoint genes, such as PD1 and CTLA4, gene disruptions in T cells can be used to prevent expression of T cell receptors that may result in off-target effects or receptors that are used for pathogen entry, such as CCR5.7 The introduction of novel functions and artificial antigen receptors into T cells has also shown promise in the cancer setting as the joint co-recipient of Science’s Breakthrough of the Year in 2013 with checkpoint inhibitors.8 Chimeric antigen receptors (CARs) provide additional T cell specificity independent of MHC and allow for potent signaling upon recognition of their ligands in diseased cells.
The promise of immunotherapy and the use of genetically modified T cells have also been used in applications outside cancer including HIV. CAR strategies for HIV have been described since the 1990s, and these trials showed that infusion of these cells were safe and that there has been long-term persistence of CAR-modified T cells in some HIV+ individuals. However, there have been concerns about the potential for HIV to infect adoptively transferred T cells (particularly since some strategies used a CD4-zeta CAR).9 The knockdown of CCR5 is a potential strategy to confer HIV resistance, especially since the sole documented patient cured of HIV was an HIV+ individual with acute myeloid leukemia (AML) who received an allogeneic stem cell transplant from a healthy donor who was homozygous for the CCR5d32 mutation. This mutation prevents infection of CCR5 strains of HIV onto CD4+ T cells because these viruses rely on CCR5 as a co-receptor for entry.10 Toward this effort, gene modifications to artificially introduce CCR5 deletions have been explored and have been shown to be safe in a phase I trial, although the anti-viral effects of the modified T cells were unclear.11 Nevertheless, results from the HIV CAR T cell studies and studies utilizing zinc finger nuclease approaches to knock down CCR5 suggest that combining these two modifications to produce a T cell therapeutic that has potent anti-HIV activity and is resistant to HIV infection is highly desirable.9
In this issue of Molecular Therapy, Hale et al.2 present a glimpse into how such a product might look. Using HDR, their work describes directed delivery of a second generation CAR based off broadly neutralizing antibodies specific for the HIV envelope glycoprotein into the CCR5 locus (see Figure 1). Their work builds off their previous approaches12, 13 using megaTAL nucleases to introduce the DNA strand breaks from which repair is initiated. They introduce their transgene through an AAV donor template and show that modified cells are capable of suppressing HIV viral replication in vitro when compared to cells transduced with a control CAR (CD19-CAR).2 Four days after coculture with peripheral blood mononuclear cells (PBMCs) containing actively replicating HIV, T cells modified with the PGT145-CAR and a CCR5 disruption had lower measurable viral particles (using the p24 ELISA as the readout) compared to T cells transduced with PGT145 CAR T cells and an intact CCR5.2
Figure 1.
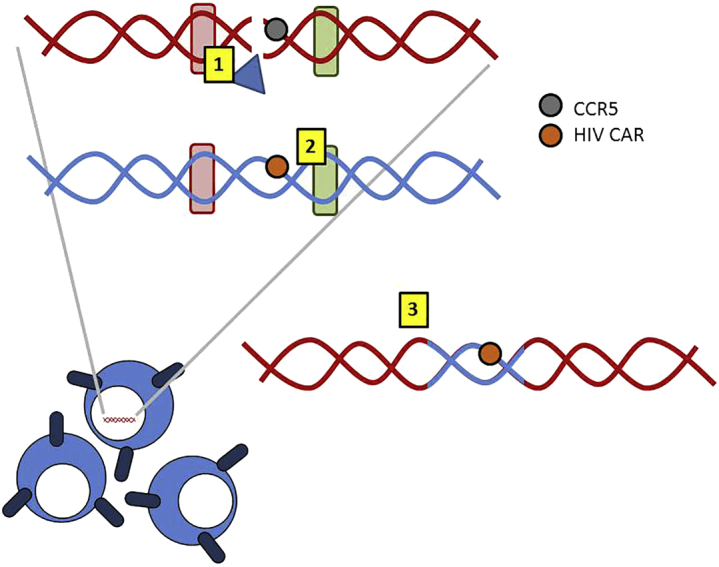
Harnessing Homology-Directed Repair for Gene Modification
Homology-directed repair (HDR), represented here, (1) employs double strand breaks introduced by the megaTAL (blue triangle) in the region of interest (i.e., CCR5) and initiates repair via the (2) homologous regions (rounded red and green rectangles) present in a donor template to (3) switch in a transgene of interest (i.e., the chimeric antigen receptor targeting HIV [HIV CAR]) during the repair process. The use of HDR to introduce constructs has the advantages of simultaneously removing a gene while introducing a new one and of directed integration (avoiding potentially “unsafe” regions).
This novel T cell therapeutic, therefore, has the potential to be resistant to HIV infection and highly potent against HIV. The caveat is that the studies presented were all carried out in vitro. However, the group was quick to point out in their discussion that the work warrants further preclinical testing in animal models. It will be interesting in ongoing studies to evaluate whether the approach can protect adoptively transferred gene-modified cells post infusion in animal models and whether it mediates a sterilizing cure. However, it is important to emphasize that optimization of such an approach to knock down an unfavorable gene (CCR5) and replace it with a favorable one (HIV-CAR) is a critical first step toward clinical translation, with applications beyond HIV, including disruption of endogenous T cell receptors (TCRs) in favor of CARs and deletion of inhibitory signals in favor of activating ones.
References
- 1.Powell A.B., Williams K., Cruz C.R. Gene-modified, cell-based therapies-an overview. Cytotherapy. 2016;18:1351–1359. doi: 10.1016/j.jcyt.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Hale M., Mesojednik T., Romano Ibarra G.S., Sahni J., Bernard A., Sommer K., Scharenberg A.M., Rawlings D.J., Wagner T.A. Engineering HIV-resistant, anti-HIV chimeric antigen receptor T cells. Mol. Ther. 2017;25:570–579. doi: 10.1016/j.ymthe.2016.12.023. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jasin M., Haber J.E. The democratization of gene editing: Insights from site-specific cleavage and double-strand break repair. DNA Repair (Amst.) 2016;44:6–16. doi: 10.1016/j.dnarep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., DeClercq J.J., Hayward S.B., Li P.W., Shivak D.A., Gregory P.D., Lee G., Holmes M.C. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 2016;44:e30. doi: 10.1093/nar/gkv1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korman A.J., Peggs K.S., Allison J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Shaffer D.R., Alvarez Arias D.A., Nakazaki Y., Pos W., Torres A.J., Cremasco V., Dougan S.K., Cowley G.S., Elpek K. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeley M., Long A. Advances in siRNA delivery to T-cells: potential clinical applications for inflammatory disease, cancer and infection. Biochem. J. 2013;455:133–147. doi: 10.1042/BJ20130950. [DOI] [PubMed] [Google Scholar]
- 8.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 9.Wagner T.A. Combining cell and gene therapy in an effort to eradicate HIV. AIDS Patient Care STDS. 2016;30:534–538. doi: 10.1089/apc.2016.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allers K., Hütter G., Hofmann J., Loddenkemper C., Rieger K., Thiel E., Schneider T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 11.Tebas P., Stein D., Tang W.W., Frank I., Wang S.Q., Lee G., Spratt S.K., Surosky R.T., Giedlin M.A., Nichol G. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romano Ibarra G.S., Paul B., Sather B.D., Younan P.M., Sommer K., Kowalski J.P., Hale M., Stoddard B., Jarjour J., Astrakhan A. Efficient modification of the CCR5 locus in primary human T cells with megaTAL nuclease establishes HIV-1 resistance. Mol. Ther. Nucleic Acids. 2016;5:e352. doi: 10.1038/mtna.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sather B.D., Romano Ibarra G.S., Sommer K., Curinga G., Hale M., Khan I.F., Singh S., Song Y., Gwiazda K., Sahni J. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci. Transl. Med. 2015;7:307ra156. doi: 10.1126/scitranslmed.aac5530. [DOI] [PMC free article] [PubMed] [Google Scholar]


