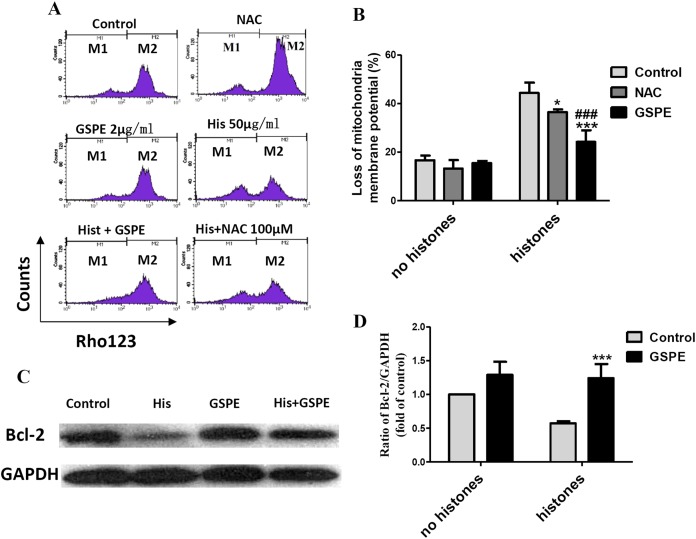
Figure 3. Grape seed proanthocyanidin extract inhibited mitochondrial damage caused by histones.
Human lymphocytes were cultured with PBS (control), NAC (100 μM), histones (His) (50 μg/ml), GSPE (2 μg/ml), histones plus GSPE, or histones (His) plus NAC. Both GSPE and NAC were used to pre-treat cells for 2 h, then histones were added and cells cultured for an additional 2.5 h. Mitochondrial membrane potential (Δψm) was detected by Rhodamine (Rho) 123 and flow cytometry analysis. (A) Representative pictures of Rho 123 fluorescence in lymphocytes in indicated groups. M1 represents the percentage of lymphocytes with Δψm loss, M2 represents the percentage of lymphocytes without Δψm loss. (B) Bar graph showing differences in the percentage of cells with Δψm loss. There was a significant interaction between the effects of histones and GSPE on Δψm loss, F = 15.31, Df = 1, P < 0.001. Df for error is 12. Values were presented as mean ±SD (n = 3). *P < 0.05, ***P < 0.001 vs control group treated with histones; ###P < 0.001 vs NAC group treated with histones. (C) Representative blots of Bcl-2 expression in lymphocytes in indicated groups. Human lymphocytes were cultured with PBS (control), histone (His) (50 μg/ml), GSPE (2 μg/ml), or histones (His) plus GSPE. GSPE was used to pre-treat cells for 2 h, then histones were added and cultured for 2.5 h. Bcl-2 expression was evaluated by western blotting. GAPDH was used as a loading control to normalize data for differences in the amount of total proteins loaded per lane. (D) Quantification of Bcl-2/GAPDH expression ratio. Densitometric analysis of relative bcl-2 band intensities were used to compare groups. Results were expressed as fold change compared to control group without histones treatment. The main effects of histones and GSPE are significant. For histones, F = 8.22, Df = 1, P < 0.05, for GSPE, F = 33.12, Df = 1, P < 0.001. Df for error is 8. Values are presented as mean ±SD (n = 3). ***P < 0.001 vs control group treated with histones.