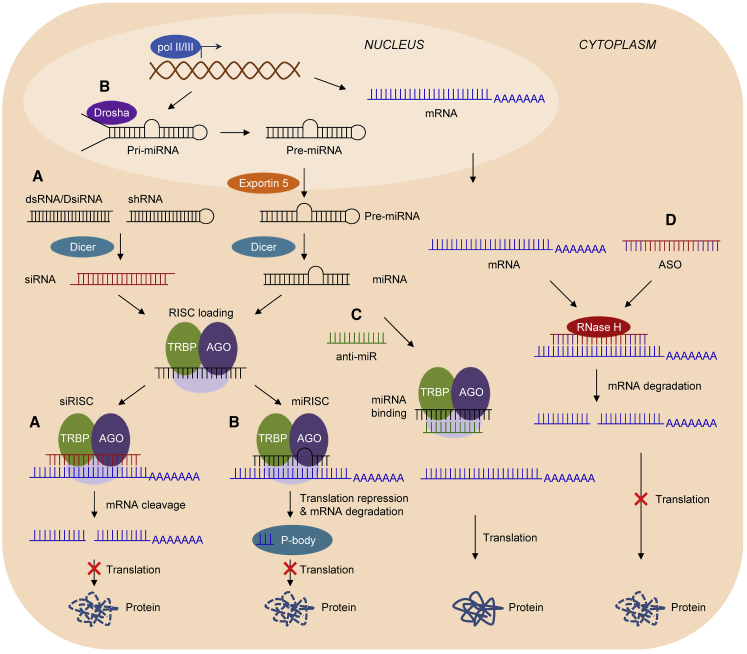
Figure 1.
Gene Regulation Pathways for siRNA, miRNA, Anti-miR, and ASO
(A and B) RNAi pathway. (A) First, miRNA genes are transcribed by RNA polymerase II or III (pol II/III) into long (60- to 100-nt) primary miRNA (pri-miRNA) sequences with stem-loop structures that are further cleaved by the Drosha–DGCR8 (DiGeorge syndrome critical region gene 8) complex to form ∼70-nt precursor miRNA (pre-miRNA) structures containing 2-nt overhangs at their 3′ ends.118, 119 After being transported to the cytoplasm by exportin-5, pre-miRNAs are processed by Dicer into mature (∼22-nt) miRNAs. (B) siRNAs can be obtained directly by chemical synthesis. They can also be generated from the cleavage of double-stranded RNA (dsRNA), DsiRNA, or short hairpin RNA (shRNA) by Dicer.120, 121 shRNA is transcribed by pol II from an shRNA-expressing plasmid. A chemically synthesized siRNA-, dsRNA-, DsiRNA-, or shRNA-expressing plasmid can be exogenously added into the cell. Then, mature miRNA and siRNA will be assembled into the RNA-induced silencing complex (RISC). RISC contains AGO (Argonaute), TRBP (HIV-1 transactivation responsive element [TAR] RNA-binding protein), and other proteins. The antisense (guide) strand of siRNA/miRNA remains in the RISC, forming activated siRISC or miRISC. Activated siRISC finds its target mRNA in a complete-match way, cleaves the target mRNA, and thus blocks its translation (A). Meanwhile, activated miRISC binds to target mRNA by forming a bulge sequence in the middle and inhibits its expression by either translation repression or mRNA cleavage (B). Translationally repressed mRNA is either stored in P bodies or enters the mRNA decay pathway for destruction. (C) When anti-miRs are added into the cell, they can specifically associate with Argonaute-bound miRNAs, preventing association with target mRNAs, which leads to increased expression of the targeted mRNAs.122 (D) ASOs commonly have a phosphorothioate backbone with flanks that are modified with 2′-O-methyl (2′-OMe) or 2′-MOE or S-cEt residues (highlighted in purple).94 Flank modifications can improve the binding affinity and nuclease resistance of ASOs and reduce immune stimulation of the PS backbone and do not support RNase H cleavage. The unmodified “gap” in a gapmer-mRNA duplex will recruit RNase H, resulting in degradation of duplexed mRNA.123